INTRODUCING PHENYLAMINEThis page looks at the structure and physical properties of phenylamine - also known as aniline or aminobenzene. Phenylamine has an -NH2 group attached directly to a benzene ring. The structure of phenylaminePhenylamine is a primary amine - a compound in which one of the hydrogen atoms in an ammonia molecule has been replaced by a hydrocarbon group. However, in comparison with simple primary amines like methylamine, the properties of phenylamine are slightly different. This is because the lone pair on the nitrogen atom interacts with the delocalised electrons in the benzene ring. The simplest way to draw the structure of phenylamine is:
|
||||||||||
|
Warning! You need to understand about the bonding in benzene in order to make sense of this next bit. If you follow this link, you may have to explore several other pages before you are ready to come back here again. Use the BACK button (or HISTORY file or GO menu) on your browser to return to this page. |
||||||||||
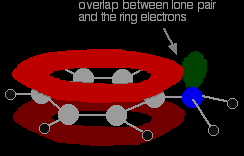
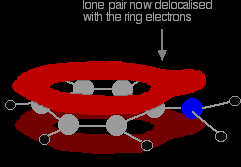
There is an interaction between the delocalised electrons in the benzene ring and the lone pair on the nitrogen atom. The lone pair overlaps with the delocalised ring electron system . . .
. . . giving a structure rather like this:
The donation of the nitrogen's lone pair into the ring system increases the electron density around the ring. That makes the ring much more reactive than it is in benzene itself. |
||||||||||
|
Note: The ring reactions of phenylamine aren't on any of the current UK A level chemistry syllabuses, so I haven't followed this up anywhere on this site. |
||||||||||
It also reduces the availability of the lone pair on the nitrogen to take part in other reactions. In particular, it makes phenylamine much more weakly basic than primary amines where the -NH2 group isn't attached to a benzene ring. That will be explored elsewhere in this section. (See the phenylamine menu - link at the bottom of this page.) Physical propertiesPure phenylamine is a colourless liquid, but it darkens rapidly on exposure to light and air. It is normally a brown oily liquid. Melting and boiling points It is useful to compare phenylamine's melting and boiling points with those of methylbenzene (toluene). Both molecules contain a similar number of electrons and are a very similar shape. That means that the intermolecular attractions due to van der Waals dispersion forces are going to be very similar. |
||||||||||
|
Note: If you aren't happy about intermolecular forces (including van der Waals dispersion forces and hydrogen bonds) then you really ought to follow this link before you go on. This section won't make much sense to you if you aren't familiar with the various sorts of intermolecular forces. Use the BACK button on your browser to return to this page. |
||||||||||
The reason for the higher values for phenylamine is in part due to permanent dipole-dipole attractions due to the electronegativity of the nitrogen - but is mainly due to hydrogen bonding. Hydrogen bonds can form between a lone pair on a nitrogen on one molecule and the hydrogen on the -NH2 group of one of its neighbours. |
||||||||||
|
Note: If you are amazingly wide-awake, you might wonder how the lone pair can form hydrogen bonds if it is delocalised into the ring electrons. The truth is that the delocalisation isn't complete. You can think of the lone pair as still being there, but not as effective as it would otherwise be. |
||||||||||
Solubility in water Phenylamine is slightly soluble in water - about 3.6 g (depending on where you get the data from!) of phenylamine will dissolve in 100 g of water at 20°C. Mixtures containing more phenylamine than this separate into two layers, with the phenylamine forming the bottom one. Phenylamine is somewhat soluble in water because of its ability to form hydrogen bonds with the water. However, the benzene rings in the phenylamine break more hydrogen bonds between water molecules than are reformed between water and the -NH2 groups. The water molecules also disrupt fairly strong van der Waals attractions between the phenylamine molecules. Both of these effects mean that dissolving phenylamine in water isn't very energetically profitable, and so stop the phenylamine from being very soluble.
|
||||||||||