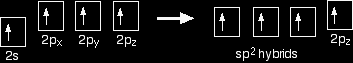BONDING IN BENZENE |
|
|
Important! This article builds on knowledge about the bonding in methane, and the bonding in ethene. You will find the current page much easier to understand if you read these other ones first. You may also find it useful to read the article on orbitals if you aren't sure about simple orbital theory. You can also read about the evidence which leads to the structure described in this article. That page includes the Kekulé structure for benzene and the reasons that it isn't very satisfactory. |
|
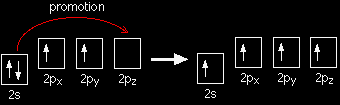
An orbital model for the benzene structureBuilding the orbital model Benzene is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1). Each carbon atom has to join to three other atoms (one hydrogen and two carbons) and doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 pair into the empty 2pz orbital. So the first thing that happens is . . . Promotion of an electron
There is only a small energy gap between the 2s and 2p orbitals, and an electron is promoted from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when these electrons are used for bonding more than compensates for the initial input. The carbon atom is now said to be in an excited state. Hybridisation Because each carbon is only joining to three other atoms, when the carbon atoms hybridise their outer orbitals before forming bonds, they only need to hybridise three of the orbitals rather than all four. They use the 2s electron and two of the 2p electrons, but leave the other 2p electron unchanged.
|
|
|
Important! If you have any doubts about this then you should follow the links at the top of the page. |
|
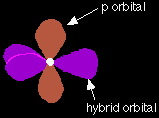
The new orbitals formed are called sp2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. The three sp2 hybrid orbitals arrange themselves as far apart as possible - which is at 120° to each other in a plane. The remaining p orbital is at right angles to them. 
Each carbon atom now looks like the diagram on the right. This is all exactly the same as happens in ethene. The difference in benzene is that each carbon atom is joined to two other similar carbon atoms instead of just one. Each carbon atom uses the sp2 hybrids to form sigma bonds with two other carbons and one hydrogen atom. The next diagram shows the sigma bonds formed, but for the moment leaves the p orbitals alone. |
|
|
Remember: A sigma bond is formed by the end-to-end overlap between atomic orbitals. |
|
Only a part of the ring is shown because the diagram gets extremely cluttered if you try to draw any more. Notice that the p electron on each carbon atom is overlapping with those on both sides of it. This extensive sideways overlap produces a system of pi bonds which are spread out over the whole carbon ring. Because the electrons are no longer held between just two carbon atoms, but are spread over the whole ring, the electrons are said to be delocalised. The six delocalised electrons go into three molecular orbitals - two in each. |
|
|
Remember: A molecular orbital is the region of space which contains a bonding pair of electrons. Warning! Be very careful how you phrase this in exams. You must never talk about the p orbitals on the carbons overlapping sideways to produce a delocalised pi bond. This upsets examiners because a pi bond can only hold 2 electrons - whereas in benzene there are 6 delocalised electrons. Talk instead about a "pi system" - or just about the delocalised electrons. |
|
In common with the great majority of descriptions of the bonding in benzene, we are only going to show one of these delocalised molecular orbitals for simplicity. 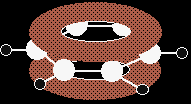
In the diagram, the sigma bonds have been shown as simple lines to make the diagram less confusing. The two rings above and below the plane of the molecule represent one molecular orbital. The two delocalised electrons can be found anywhere within those rings. The other four delocalised electrons live in two similar (but not identical) molecular orbitals. Relating the orbital model to the properties of benzene |
|
|
Note: To get the best out of this section you ought to read the article on the Kekulé structure for benzene. |
|
The shape of benzene Benzene is a planar regular hexagon, with bond angles of 120°. This is easily explained. It is a regular hexagon because all the bonds are identical. The delocalisation of the electrons means that there aren't alternating double and single bonds. It is planar because that is the only way that the p orbitals can overlap sideways to give the delocalised pi system. The energetic stability of benzene This is accounted for by the delocalisation. As a general principle, the more you can spread electrons around - in other words, the more they are delocalised - the more stable the molecule becomes. The extra stability of benzene is often referred to as "delocalisation energy". The reluctance of benzene to undergo addition reactions With the delocalised electrons in place, benzene is about 150 kJ mol-1 more stable than it would otherwise be. If you added other atoms to a benzene ring you would have to use some of the delocalised electrons to join the new atoms to the ring. That would disrupt the delocalisation and the system would become less stable. Since about 150 kJ per mole of benzene would have to be supplied to break up the delocalisation, this isn't going to be an easy thing to do. The symbol for benzene 
Although you will still come across the Kekulé structure for benzene, for most purposes we use the structure on the right. The hexagon shows the ring of six carbon atoms, each of which has one hydrogen attached. (You have to know that - counting bonds to find out how many hydrogens to add doesn't work in this particular case.) The circle represents the delocalised electrons. It is essential that you include the circle. If you miss it out, you are drawing cyclohexane and not benzene.
|
|