EXPLAINING THE NUCLEOPHILIC SUBSTITUTION REACTIONS BETWEEN HALOGENOALKANES AND AMMONIA
This page guides you through the nucleophilic substitution mechanisms for the reactions between halogenoalkanes and ammonia to produce primary amines. It deals only with primary and tertiary halogenoalkanes. No current UK-based syllabus for 16 - 18 year olds is likely to ask about the reaction between secondary halogenoalkanes and ammonia. (It's not difficult - it's just not there!) If you are interested in further substitution reactions, at the bottom of this page you will find a link to a separate page dealing with these. |
|
|
Important! It would help if you first read the page What is nucleophilic substitution? before you go on. You must also be clear about the differences between primary, secondary and tertiary halogenoalkanes. |
|
The reaction between primary halogenoalkanes and ammonia - the SN2 mechanismAmmonia as a nucleophile A nucleophile is a species (an ion or a molecule) which is strongly attracted to a region of positive charge in something else. Nucleophiles are either fully negative ions, or else have a strongly 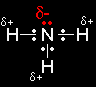
Ammonia obviously doesn't carry a negative charge. However, nitrogen is more electronegative than hydrogen, and so the nitrogen atom carries some degree of negative charge. It also has an active lone pair of electrons. The attack on the |
|
|
Note: If you aren't sure about electronegativity follow this link before you read on. Use the BACK button on your browser to return to this page. |
|
The nucleophilic substitution reaction - an SN2 reaction 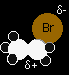
We'll talk this reaction through with bromoethane as a typical primary halogenoalkane. The bromoethane has a polar bond between the carbon and the bromine. The lone pair on the nitrogen will be strongly attracted to the The movement goes on until the ammonia is firmly attached to the carbon, and the bromine has been expelled as a Br- ion.
Notice that the nitrogen in the product ion carries a positive charge (highlighted in red to draw attention to it). That charge has to be there for two reasons:
|
|
|
Note: Nitrogen with a positive charge has the same arrangement of electrons as a carbon atom - which normally forms 4 bonds. |
|
|
Technically, this is known as an SN2 reaction. S stands for substitution, N for nucleophilic, and the 2 is because the initial stage of the reaction involves two species - the bromoethane and the ammonia. If your syllabus doesn't refer to SN2 reactions by name, you can just call it nucleophilic substitution. The product of this reaction is a salt called ethylammonium bromide. Ethylammonium bromide is a salt of a primary amine and the acid, HBr. A primary amine has the formula R-NH2. It is primary in the sense that there is only one alkyl group attached to the nitrogen atom. Primary amines are weak bases very similar to ammonia and so form salts with acids. For example, ammonia reacts with HBr to give ammonium bromide, NH4+ Br-. The salt in the equation above is the one formed from ethylamine, CH3CH2NH2, and HBr. At this point you need to know whether your examiners are happy for you to stop there, or want you to go a stage further to form the free primary amine. |
|
|
Note: You need to check the mark schemes for recent exam papers, or any support material published by your examiners. You won't be able to tell this by looking at your syllabus, or at the exam papers themselves. You can find out how to get hold of this material by visiting your examiners' web site. If you are working to a UK-based syllabus for 16 - 18 year olds, you can find a link to this on the syllabuses page. |
|
If they want the second step, an ammonia molecule now removes a hydrogen ion from the nitrogen.
The organic product is ethylamine, CH3CH2NH2. Notice that this change is reversible. The curly arrows for the reverse change haven't been shown to avoid confusion, but you can easily picture the lone pair on the nitrogen reclaiming its hydrogen from the NH4+ ion. What you will end up with is a mixture of all four of the species in this last equation - together, of course, with the bromide ions formed in the first stage. The higher the proportion of ammonia in the original reaction mixture, the greater the chance of the free ethylamine being formed. The reaction between tertiary halogenoalkanes and ammonia - the SN1 mechanism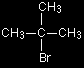
We'll talk this mechanism through with a simple tertiary halogenoalkane like the one on the right (2-bromo-2-methylpropane). Why do tertiary halogenoalkanes need a different mechanism? When a nucleophile attacks a primary halogenoalkane, it approaches the With a tertiary halogenoalkane, this approach from the back is impossible. The back of the molecule is completely cluttered with CH3 groups. The SN1 mechanism In the first stage, a small proportion of the halogenoalkane ionises to give a carbocation (carbonium ion) and a bromide ion.
This reaction is possible because tertiary carbocations are relatively stable compared with secondary or primary ones. Even so, the reaction is slow. |
|
|
Note: Not sure about the stability of carbocations (carbonium ions)? Follow this link if you need to find out. |
|
Once the carbocation is formed, however, it would react immediately it came into contact with an ammonia molecule. The lone pair on the nitrogen is strongly attracted towards the positive carbon, and moves towards it to create a new bond.
How fast the reaction happens overall is going to be governed by how fast the halogenoalkane ionises - because that's a slow process. Because this initial slow step only involves one species, the mechanism is described as SN1 - substitution, nucleophilic, one species taking part in the initial slow step. A salt is formed, and again you need to know whether your examiners want you to go on beyond this to the formation of the free amine. If they do, one of the hydrogens attached to the nitrogen is removed by another ammonia molecule.
|
|




