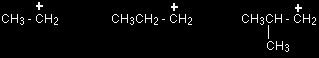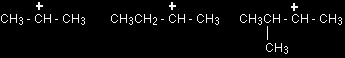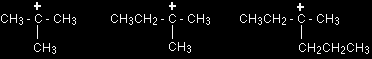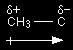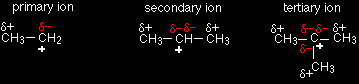CARBOCATIONS (or CARBONIUM IONS)All carbocations (previously known as carbonium ions) carry a positive charge on a carbon atom. The name tells you that - a cation is a positive ion, and the "carbo" bit refers to a carbon atom. However there are important differences in the structures of various types of carbocations. The different kinds of carbocationsPrimary carbocations In a primary (1°) carbocation, the carbon which carries the positive charge is only attached to one other alkyl group. |
||
|
Help! An alkyl group is a group such as methyl, CH3, or ethyl, CH3CH2. These are groups containing chains of carbon atoms which may be branched. Alkyl groups are given the general symbol R. |
||
Some examples of primary carbocations include:
Notice that it doesn't matter how complicated the attached alkyl group is. All you are doing is counting the number of bonds from the positive carbon to other carbon atoms. In all the above cases there is only one such link. 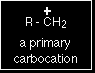
Using the symbol R for an alkyl group, a primary carbocation would be written as in the box. Secondary carbocations In a secondary (2°) carbocation, the carbon with the positive charge is attached to two other alkyl groups, which may be the same or different. Examples:
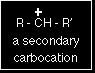
A secondary carbocation has the general formula shown in the box. R and R' represent alkyl groups which may be the same or different. Tertiary carbocations In a tertiary (3°) carbocation, the positive carbon atom is attached to three alkyl groups, which may be any combination of same or different.
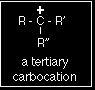
A tertiary carbocation has the general formula shown in the box. R, R' and R" are alkyl groups and may be the same or different. The stability of the various carbocationsThe "electron pushing effect" of alkyl groups You are probably familiar with the idea that bromine is more electronegative than hydrogen, so that in a H-Br bond the electrons are held closer to the bromine than the hydrogen. A bromine atom attached to a carbon atom would have precisely the same effect - the electrons being pulled towards the bromine end of the bond. The bromine has a negative inductive effect. |
||
|
Help! If you aren't familiar with all of this, follow this link to read about electronegativity and bond polarity before you go any further. Use the BACK button on your browser to return to this page. |
||
Alkyl groups do precisely the opposite and, rather than draw electrons towards themselves, tend to "push" electrons away. |
||
|
Note: The term "electron pushing" is only to help remember what happens. The alkyl group doesn't literally "push" the electrons away - the other end of the bond attracts them more strongly. |
||
This means that the alkyl group becomes slightly positive ( This is sometimes shown as, for example:
The arrow shows the electrons being "pushed" away from the CH3 group. The plus sign on the left-hand end of it shows that the CH3 group is becoming positive. The symbols The importance of spreading charge around in making ions stable The general rule-of-thumb is that if a charge is very localised (all concentrated on one atom) the ion is much less stable than if the charge is spread out over several atoms. Applying that to carbocations of various sorts . . .
You will see that the electron pushing effect of the CH3 group is placing more and more negative charge on the positive carbon as you go from primary to secondary to tertiary carbocations. The effect of this, of course, is to cut down that positive charge. At the same time, the region around the various CH3 groups is becoming somewhat positive. The net effect, then, is that the positive charge is being spread out over more and more atoms as you go from primary to secondary to tertiary ions. The more you can spread the charge around, the more stable the ion becomes.
|
||
|
Note: The symbol "<" means "is less than". So what this is saying is that primary ions are less stable than secondary ones which in turn are less stable than tertiary ones. |
||
The stability of the carbocations in terms of energetics 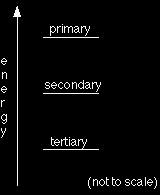
When we talk about secondary carbocations being more stable than primary ones, what exactly do we mean? We are actually talking about energetic stability - secondary carbocations are lower down an energy "ladder" than primary ones. This means that it is going to take more energy to make a primary carbocation than a secondary one. If there is a choice between making a secondary ion or a primary one, it will be much easier to make the secondary one. Similarly, if there is a choice between making a tertiary ion or a secondary one, it will be easier to make the tertiary one. This has important implications in the reactions of unsymmetrical alkenes. If you are interested in these, follow the link below to the electrophilic addition reactions menu.
|
||