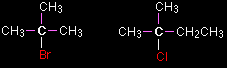HALOGENOALKANESHalogenoalkanes are also called haloalkanes or alkyl halides. All halogenoalkanes contain a halogen atom - fluorine, chlorine, bromine or iodine - attached to an alkyl group. |
|
|
Note: An alkyl group is a group such as methyl, CH3, or ethyl, CH3CH2. These are groups containing chains of carbon atoms which may be branched. Alkyl groups are given the general symbol R. |
|
The different kinds of halogenoalkanesPrimary halogenoalkanes In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group. Some examples of primary halogenoalkanes include:
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage from the CH2 group holding the halogen to an alkyl group. There is an exception to this. CH3Br and the other methyl halides are often counted as primary halogenoalkanes even though there are no alkyl groups attached to the carbon with the halogen on it. Secondary halogenoalkanes In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. Examples:
Tertiary halogenoalkanes In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
|
|