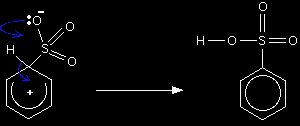THE SULPHONATION OF BENZENE
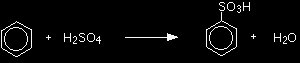
This page gives you the facts and a simple, uncluttered mechanism for the electrophilic substitution reaction between benzene and sulphuric acid (or sulphur trioxide). If you want this mechanism explained to you in detail, there is a link at the bottom of the page. The electrophilic substitution reaction between benzene and sulphuric acidThe facts There are two equivalent ways of sulphonating benzene:
Or:
The product is benzenesulphonic acid. The electrophile is actually sulphur trioxide, SO3, and you may find the equation for the sulphonation reaction written:
|
|
|
Note: Which version of this equation you use will depend on what question you are being asked. If the question refers to the reaction with sulphuric acid, then you must use that one. If the question refers to SO3 as the electrophile, then you could use this one. |
|
The formation of the electrophile The sulphur trioxide electrophile arises in one of two ways depending on which sort of acid you are using. Concentrated sulphuric acid contains traces of SO3 due to slight dissociation of the acid.
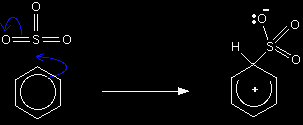
Fuming sulphuric acid, H2S2O7, can be thought of as a solution of SO3 in sulphuric acid - and so is a much richer source of the SO3. Sulphur trioxide is an electrophile because it is a highly polar molecule with a fair amount of positive charge on the sulphur atom. It is this which is attracted to the ring electrons. The electrophilic substitution mechanism Stage one
Stage two
The second stage of the reaction involves a transfer of the hydrogen from the ring to the negative oxygen.
|
|