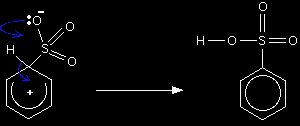EXPLAINING THE SULPHONATION OF BENZENE
This page guides you through the mechanism for the electrophilic substitution reaction between benzene and sulphuric acid (or sulphur trioxide) The electrophilic substitution reaction between benzene and sulphuric acidThe formation of the electrophile The electrophile is sulphur trioxide, and this arises in one of two ways depending on which sort of acid you are using. Concentrated sulphuric acid contains traces of SO3 due to slight dissociation of the acid.
|
|
|
Dissociation: This is a reversible splitting up of a compound. In this case, the sulphuric acid splits into water and SO3, and at the same time these combine back together again to make sulphuric acid. The overall effect is that concentrated sulphuric acid contains small amounts of SO3. |
|
Fuming sulphuric acid, H2S2O7, can be thought of as a solution of SO3 in sulphuric acid - and so is a much richer source of the SO3. |
|
|
Note: You could think of the formula as being essentially H2SO4.SO3. |
|
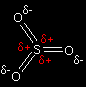
Although sulphur trioxide isn't ionic, it is highly polar. The three oxygens are more electronegative than the sulphur and so draw electrons towards themselves. That leaves the sulphur atom fairly positively charged. It is this |
|
|
Note: If you aren't sure about electronegativity and polar bonds you might like to follow this link. Use the BACK button on your browser to return to this page. |
|
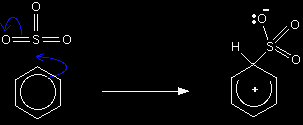
The electrophilic substitution mechanism Stage one Two electrons from the delocalised system are used to form a new bond with the slightly positive sulphur atom. Because those two electrons aren't a part of the delocalised system any longer, the delocalisation is partly broken, and in the process the ring gains a positive charge. To make room for the new bond between the ring and the sulphur, two of the electrons joining the sulphur to one of the oxygens are forced right out on to the oxygen atom, giving it a negative charge.
The hydrogen shown on the ring is the one which was already attached to that top carbon atom. We need to show it there because it has to be removed in the second stage. |
|
|
Note: If you aren't sure about the use of curly arrows follow this link before you go on. Use the BACK button on your browser to return to this page. |
|
Stage two This second step is different from all the other benzene electrophilic substitution reactions you might have already looked at on this site. This time there isn't a separate negative ion to remove the hydrogen atom from the ring. Instead it is removed by a lone pair on the negative oxygen atom.
The lone pair forms a bond with the hydrogen atom, releasing the electrons in the hydrogen-to-ring bond so that they can re-establish the delocalisation. This particular mechanism needs to be drawn with rather more thought than the other electrophilic substitution mechanisms. In particular, you have to make sure that you put the negative charge on the right oxygen in the intermediate ion. It must be the one which is closest to the hydrogen you intend to take off the ring, otherwise there is no way of drawing sensible curly arrows in the second stage!
To menu of electrophilic substitution reactions. . . |
|