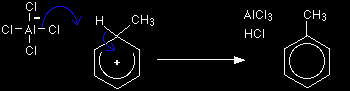THE FRIEDEL-CRAFTS ALKYLATION OF BENZENE
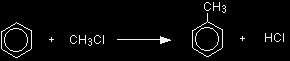
This page gives you the facts and a simple, uncluttered mechanism for the electrophilic substitution reaction between benzene and chloromethane in the presence of an aluminium chloride catalyst. Any other chloroalkane would work similarly. If you want the Friedel-Crafts alkylation mechanism explained to you in detail, there is a link at the bottom of the page. The electrophilic substitution reaction between benzene and chloromethaneWhat is alkylation? Alkylation means substituting an alkyl group into something - in this case into a benzene ring. A hydrogen on the ring is replaced by a group like methyl or ethyl and so on. The facts Benzene is treated with a chloroalkane (for example, chloromethane or chloroethane) in the presence of aluminium chloride as a catalyst. On this page, we will look at substituting a methyl group, but any other alkyl group could be used in the same way. Substituting a methyl group gives methylbenzene - once known as toluene.
or better:
The aluminium chloride isn't written into these equations because it is acting as a catalyst. If you wanted to include it, you could write AlCl3 over the top of the arrow. |
|
|
Note: The methylbenzene formed is more reactive than the original benzene, and so the reaction doesn't stop there. You get further methyl groups substituted around the ring. You won't have to worry about this for A' level. |
|
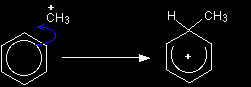
The formation of the electrophile The electrophile is CH3+. It is formed by reaction between the chloromethane and the aluminium chloride catalyst.
The electrophilic substitution mechanism Stage one
Stage two
The hydrogen is removed by the AlCl4- ion which was formed at the same time as the CH3+ electrophile. The aluminium chloride catalyst is re-generated in this second stage. |
|