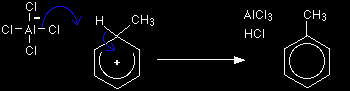EXPLAINING THE FRIEDEL-CRAFTS ALKYLATION OF BENZENE
This page guides you through the mechanism for the Friedel-Crafts alkyation of benzene involving an electrophilic substitution reaction between benzene and a chloroalkane like chloromethane in the presence of an aluminium chloride catalyst. |
|
|
Important! It would help if you first read the page What is electrophilic substitution? before you went on. |
|
The electrophilic substitution reaction between benzene and chloromethaneThe formation of the electrophile If you are going to replace a hydrogen atom in a benzene ring by CH3, then the electrophile must be the ion CH3+. |
|
|
Note: If you don't understand why the electrophile has got to be CH3+, then you really should look at What is electrophilic substitution? before you go on. If you are going to substitute X onto the ring, then the electrophile must be X+. If you are going to insert an CH3 group onto the ring, then the electrophile must be CH3+. |
|
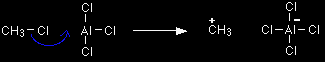
Aluminium chloride, AlCl3, is an electron deficient molecule. It is covalently bonded, but because the aluminium is only forming 3 bonds, and has no lone pairs, there are only 6 electrons around the aluminium atom rather than 8. It takes a chlorine (as a chloride ion) from the chloromethane, and forms a co-ordinate (dative covalent) bond with it.
|
|
|
Help! A co-ordinate bond is a covalent bond in which both electrons originally came from the same atom. In this case, both the electrons in the bond come from the chlorine being removed from the chloromethane. |
|
The equation simplified
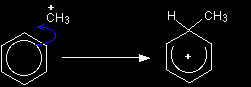
The electrophilic substitution mechanism Stage one As the CH3+ ion approaches the delocalised electrons in the benzene, those electrons are strongly attracted towards the positive charge. Two electrons from the delocalised system are used to form a new bond with the CH3+ ion. Because those two electrons aren't a part of the delocalised system any longer, the delocalisation is partly broken, and in the process the ring gains a positive charge.
The hydrogen shown on the ring is the one which was already attached to that top carbon atom. We need to show it there because it has to be removed in the second stage. Stage two The second stage involves the AlCl4-, which was produced at the same time as the CH3+ ion.
One of the aluminium-chlorine bonds breaks and both electrons from it are used to join to the hydrogen. This removes the hydrogen from the ring to form HCl, and re-generates the aluminium chloride catalyst in the process. The electrons which originally joined the hydrogen to the ring are now used to re-establish the delocalised system.
To menu of electrophilic substitution reactions. . . |
|