ELIMINATION VERSUS SUBSTITUTION IN HALOGENOALKANES
This page discusses the factors that decide whether halogenoalkanes undergo elimination reactions or nucleophilic substitution when they react with hydroxide ions from, say, sodium hydroxide or potassium hydroxide. Details for each of these types of reaction are given elsewhere, and you will find links to them from this page. The reactions Both reactions involve heating the halogenoalkane under reflux with sodium or potassium hydroxide solution. Nucleophilic substitution The hydroxide ions present are good nucleophiles, and one possibility is a replacement of the halogen atom by an -OH group to give an alcohol via a nucleophilic substitution reaction.
In the example, 2-bromopropane is converted into propan-2-ol. |
|||||||||
|
Note: If you want to read about nucleophilic substitution in this reaction in detail, follow this link. |
|||||||||
Elimination Halogenoalkanes also undergo elimination reactions in the presence of sodium or potassium hydroxide.
The 2-bromopropane has reacted to give an alkene - propene. Notice that a hydrogen atom has been removed from one of the end carbon atoms together with the bromine from the centre one. In all simple elimination reactions the things being removed are on adjacent carbon atoms, and a double bond is set up between those carbons. |
|||||||||
|
Note: If you want to read about elimination in this reaction in detail, follow this link. |
|||||||||
What decides whether you get substitution or elimination? The reagents you are using are the same for both substitution or elimination - the halogenoalkane and either sodium or potassium hydroxide solution. In all cases, you will get a mixture of both reactions happening - some substitution and some elimination. What you get most of depends on a number of factors. The type of halogenoalkane This is the most important factor.
|
|||||||||
|
Important! If you aren't clear about the various types of halogenoalkanes, it is essential that you follow this link before you read on. Use the BACK button on your browser to return to this page. |
|||||||||
For example, whatever you do with tertiary halogenoalkanes, you will tend to get mainly the elimination reaction, whereas with primary ones you will tend to get mainly substitution. However, you can influence things to some extent by changing the conditions. The solvent The proportion of water to ethanol in the solvent matters.
The temperature Higher temperatures encourage elimination. Concentration of the sodium or potassium hydroxide solution Higher concentrations favour elimination. In summary For a given halogenoalkane, to favour elimination rather than substitution, use:
|
|||||||||
|
Note: The explanations for these effects are well beyond the demands of UK A level syllabuses. Some things you just have to know! |
|||||||||
The role of the hydroxide ions The role of the hydroxide ion in a substitution reaction 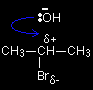
In the substitution reaction between a halogenoalkane and OH- ions, the hydroxide ions are acting as nucleophiles. For example, one of the lone pairs on the oxygen can attack the slightly positive carbon. This leads on to the loss of the bromine as a bromide ion, and the -OH group becoming attached in its place. The role of the hydroxide ion in an elimination reaction 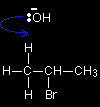
Hydroxide ions have a very strong tendency to combine with hydrogen ions to make water - in other words, the OH- ion is a very strong base. In an elimination reaction, the hydroxide ion hits one of the hydrogen atoms in the CH3 group and pulls it off. This leads to a cascade of electron pair movements resulting in the formation of a carbon-carbon double bond, and the loss of the bromine as Br-. |
|||||||||
|
Note: The competition between substitution and elimination (including the conditions needed and the mechanisms for both) is a rich source of exam questions if your syllabus includes it. You will probably find that the questions centre around secondary halogenoalkanes like 2-bromopropane, because these can easily be persuaded to do either reaction. Important: If the elimination mechanism is on your syllabus, you are quite likely to be asked questions in which the substitution reaction crops up as well. If you need to revise this in detail, now is a good time to do it. |
|||||||||
To menu of elimination reactions. . . |
|||||||||