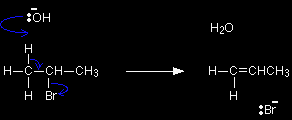THE ELIMINATION REACTIONS PRODUCING ALKENES FROM SIMPLE HALOGENOALKANES
This page gives you the facts and a simple, uncluttered mechanism for the elimination reaction between a simple halogenoalkane like 2-bromopropane and hydroxide ions (from, for example, sodium hydroxide) to give an alkene like propene. If you want the mechanism explained to you in detail, there is a link at the bottom of the page. You will also find a link to a page on elimination from more complicated halogenoalkanes where more than one product may be formed. Exam questions on this topic frequently ask about another possibility in the reactions between halogenoalkanes and hydroxide ions - nucleophilic substitution. There is also a link to a page discussing this. |
|
|
Note: The competition between substitution and elimination (including the conditions needed and the mechanisms for both) is a rich source of exam questions if your syllabus includes it. You will probably find that the questions centre around secondary halogenoalkanes like 2-bromopropane, because these can easily be persuaded to do either reaction. That's why this page deals exclusively with 2-bromopropane. |
|
The elimination reaction involving 2-bromopropane and hydroxide ionsThe facts 2-bromopropane is heated under reflux with a concentrated solution of sodium or potassium hydroxide in ethanol. Heating under reflux involves heating with a condenser placed vertically in the flask to avoid loss of volatile liquids. Propene is formed and, because this is a gas, it passes through the condenser and can be collected.
Everything else present (including anything formed in the alternative substitution reaction) will be trapped in the flask. The mechanism In elimination reactions, the hydroxide ion acts as a base - removing a hydrogen as a hydrogen ion from the carbon atom next door to the one holding the bromine. The resulting re-arrangement of the electrons expels the bromine as a bromide ion and produces propene.
|
|