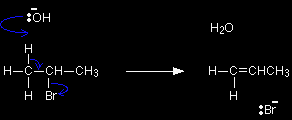EXPLAINING THE ELIMINATION REACTIONS PRODUCING ALKENES FROM SIMPLE HALOGENOALKANES
This page guides you through the elimination mechanism for the reaction between simple halogenoalkanes like 2-bromopropane and hydroxide ions from, for example, sodium hydroxide. Elimination involving more complicated halogenoalkanes and the competition between elimination and substitution in these reactions are dealt with on separate pages. These are essential parts of this topic, and you should follow the links at the bottom of this page if you haven't already done so. The elimination reaction involving 2-bromopropane and hydroxide ionsThe role of the OH- ion in an elimination reaction 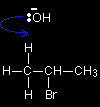
Hydroxide ions have a very strong tendency to combine with hydrogen ions to make water - in other words, the OH- ion is a very strong base. In an elimination reaction, the hydroxide ion hits one of the hydrogen atoms in the CH3 group and pulls it off. This leads to a cascade of electron pair movements resulting in the formation of a carbon-carbon double bond, and the loss of the bromine as Br-. |
|
|
Note: If you aren't happy about the use of curly arrows in mechanisms, follow this link before you go on. Use the BACK button on your browser to return to this page. |
|
The complete elimination mechanism
The attack could equally well have been on any of the other hydrogens on the left-hand carbon, or on any on the right-hand one - it simply depends on what the OH- ion hit. |
|
|
Taking chemistry further: The mechanism being described here is known as an E2 mechanism. The E stands for elimination, and the 2 is because the initial slow part of the reaction involves 2 species. This mechanism is used by primary and secondary halogenoalkanes. There is also an E1 mechanism which would apply to tertiary halogenoalkanes. (Secondary ones will also do it to some extent.) No current UK A level syllabus (or its equivalent) is likely to ask you about that. |
|