EXPLAINING THE REACTION BETWEEN UNSYMMETRICAL ALKENES AND SULPHURIC ACIDThis page guides you through the mechanism for the electrophilic addition of sulphuric acid to unsymmetrical alkenes like propene. The electrophilic addition reaction between propene and sulphuric acid |
|
|
Important! The reaction between propene and sulphuric acid has exactly the same mechanism as the one involving hydrogen bromide. It just looks more complicated because of the structure of sulphuric acid. Make sure that you understand the mechanism for the reaction between propene and HBr before you read on. It is also essential that you read about the reaction between ethene and sulphuric acid before you worry about the additional problem caused by an unsymmetrical alkene like propene. |
|
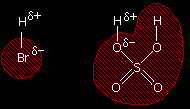
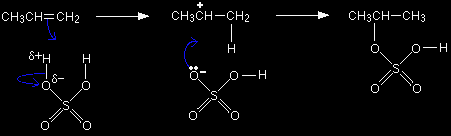
The structure of sulphuric acid Compare the structure of sulphuric acid with that of hydrogen bromide:
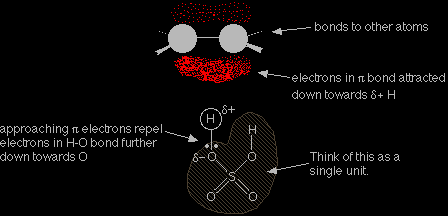
We are focussing on only one of the hydrogens in the sulphuric acid because the other one will be pointing away from the double bond in the alkene as the molecules approach each other. In each case, the hydrogen is attached to a more electronegative element, and so carries a slight positive charge. That means that the hydrogen atoms will serve as electrophiles. When the sulphuric acid reacts, the whole of the shaded part of the molecule remains as a complete unit. What happens to that unit is exactly the same as happens to the bromine in the reaction involving HBr. The mechanism Remember that the active part of the double bond is the pi bond which lies in an orbital above and below the plane of the rest of the molecule. The pi bond is very vulnerable to attack. |
|
|
Note: If this isn't fairly obvious to you, you really ought to read the page introducing electrophilic addition before you go on. Use the BACK button on your browser to return to this page. |
|
The slightly positive hydrogen atom in the sulphuric acid acts as an electrophile, and is strongly attracted to the electrons in the pi bond. As the sulphuric acid approaches the pi bond, the electrons in that bond are drawn down towards the slightly positive hydrogen atom. That repels the electrons in the hydrogen-oxygen bond down towards the oxygen.
The electron movements continue until a new bond is made between one of the carbon atoms and the hydrogen. The oxygen now has both electrons from the H-O bond, and so becomes negatively charged.
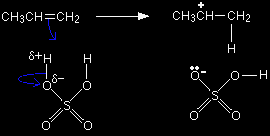
The problem is that there are two possible ways that the pi bond electrons could move. They could form a bond between the hydrogen and the left-hand carbon:
or they could form a bond with the right-hand one:
It's the second of these changes that happens more readily. In that case, a secondary carbocation is formed - and that's more energetically stable than the primary one formed in the first possibility. Because the secondary ion is more energetically stable, it will form more easily and so the reaction needs less activation energy - and so happens faster. |
|
|
Important! If you don't understand about the structure and stability of carbocations (previously known as carbonium ions) follow this link. |
|
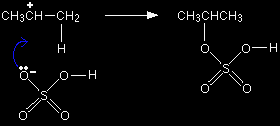
Once the ions have been formed, the lone pair on the negative oxygen is strongly attracted towards the positive carbon atom. It moves towards it and forms a bond.
That leaves you with the over-all mechanism:
|
|