THE REACTION BETWEEN SYMMETRICAL ALKENES AND SULPHURIC ACID
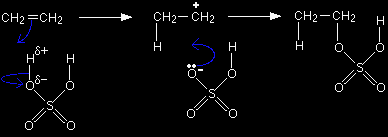
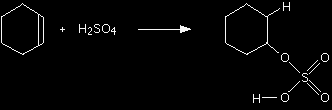
This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between sulphuric acid and alkenes like ethene and cyclohexene. If you want the mechanisms explained to you in detail, there is a link at the bottom of the page. The electrophilic addition reaction between ethene and sulphuric acidThe facts Alkenes react with concentrated sulphuric acid in the cold to produce alkyl hydrogensulphates. Ethene reacts to give ethyl hydrogensulphate.
The structure of the product molecule is sometimes written as CH3CH2HSO4, but the version in the equation is better because it shows how all the atoms are linked up. You may also find it written as CH3CH2OSO3H. 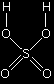
Confused by all this? Don't be! All you need to do is to learn the structure of sulphuric acid, and after that the mechanism is exactly the same as the one with hydrogen bromide. As you will find out, the formula of the product follows from the mechanism in an inevitable way. |
|
|
Important! Learn this structure for sulphuric acid. Sketch it over and over again until you can't possibly get it wrong. |
|
The mechanism for the reaction between ethene and sulphuric acid Sulphuric acid as an electrophile The hydrogen atoms are attached to very electronegative oxygen atoms which means that the hydrogens will have a slight positive charge while the oxygens will be slightly negative. In the mechanism, we just focus on one of the hydrogen to oxygen bonds, because the other one is too far from the carbon-carbon double bond to be involved in any way. The mechanism
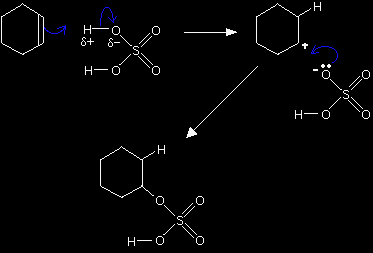
Look carefully at the structure of the product so that you can see how it relates to the various formulae given earlier (CH3CH2OSO2OH etc). The electrophilic addition reaction between cyclohexene and sulphuric acidThis time we are going straight for the mechanism without producing an initial equation. This is to show that you can work out the structure of obscure products provided you can write the mechanism.
The mechanism for the reaction between cyclohexene and sulphuric acid
Having worked out the structure of the product, you could then write a simple equation for the reaction if you wanted to.
|
|