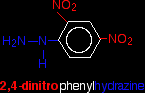
ADDITION-ELIMINATION REACTIONS OF ALDEHYDES AND KETONESThis page looks at the reaction of aldehydes and ketones with 2,4-dinitrophenylhydrazine (Brady's reagent) as a test for the carbon-oxygen double bond. It also looks briefly at some other similar reactions which are all known as addition-elimination (or condensation) reactions. The reaction with 2,4-dinitrophenylhydrazine2,4-dinitrophenylhydrazine is often abbreviated to 2,4-DNP or 2,4-DNPH. A solution of 2,4-dinitrophenylhydrazine in a mixture of methanol and sulphuric acid is known as Brady's reagent. What is 2,4-dinitrophenylhydrazine? Although the name sounds complicated, and the structure looks quite complicated, it is actually very easy to work out. Start with the formula of hydrazine. That's almost all you need to remember! Hydrazine is:
In phenylhydrazine, one of the hydrogens is replaced by a phenyl group, C6H5. This is based on a benzene ring.
|
|
|
Note: I'm using the common symbol for a benzene ring. If you aren't familiar with this, there is a carbon atom at each corner of the hexagon, together with a hydrogen atom if there isn't anything else attached. The circle in the middle of the hexagon suggests the delocalised electrons. If you want to read more about the structure of benzene, you could follow this link to another part of the site. It isn't important for the purposes of the present page, and you might have to explore quite a lot of other pages to help you to understand that one. If you do choose to follow this link, use the BACK button (or HISTORY file or GO menu if you get seriously waylaid) on your browser to return to this page. |
|
In 2,4-dinitrophenylhydrazine, there are two nitro groups, NO2, attached to the phenyl group in the 2- and 4- positions. The corner with the nitrogen attached is counted as the number 1 position, and you just number clockwise around the ring.
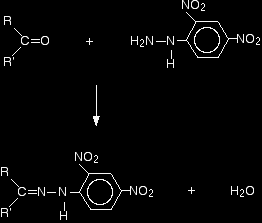
Doing the reaction Details vary slightly depending on the nature of the aldehyde or ketone, and the solvent that the 2,4-dinitrophenylhydrazine is dissolved in. Assuming you are using Brady's reagent (a solution of the 2,4-dinitrophenylhydrazine in methanol and sulphuric acid): Add either a few drops of the aldehyde or ketone, or possibly a solution of the aldehyde or ketone in methanol, to the Brady's reagent. A bright orange or yellow precipitate shows the presence of the carbon-oxygen double bond in an aldehyde or ketone. This is the simplest test for an aldehyde or ketone. The chemistry of the reaction The overall reaction is given by the equation:
R and R' can be any combination of hydrogen or hydrocarbon groups (such as alkyl groups). If at least one of them is a hydrogen, then the original compound is an aldehyde. If both are hydrocarbon groups, then it is a ketone. Look carefully at what has happened.
Provided you take care to draw the two starting molecules lined up right, working out the structure of the product is easy. The product is known as a "2,4-dinitrophenylhydrazone". Notice that all that has changed is the ending from "-ine" to "-one". That's possibly confusing! The product from the reaction with ethanal would be called ethanal 2,4-dinitrophenylhydrazone; from propanone, you would get propanone 2,4-dinitrophenylhydrazone - and so on. That's not too difficult! The reaction is known as a condensation reaction. A condensation reaction is one in which two molecules join together with the loss of a small molecule in the process. In this case, that small molecule is water. In terms of mechanisms, this is a nucleophilic addition-elimination reaction. The 2,4-dinitrophenylhydrazine first adds across the carbon-oxygen double bond (the addition stage) to give an intermediate compound which then loses a molecule of water (the elimination stage). |
|
|
Note: This mechanism isn't required by any of the UK A level syllabuses, and so you won't find it anywhere on this site. Sorry! |
|
Using the reaction The reaction has two uses in testing for aldehydes and ketones.
Some other similar reactionsIf you go back and look at the equations, nothing in the 2,4-dinitrophenylhydrazine changes during the reaction apart from the -NH2 group. You can get a similar reaction if the -NH2 group is attached to other things. In each case, the reaction would look like this:
In what follows, all that changes is the nature of the "X". with hydrazine
The product is a "hydrazone". If you started from propanone, it would be propanone hydrazone. with phenylhydrazine
The product is a "phenylhydrazone". with hydroxylamine
The product is an "oxime" - for example, ethanal oxime.
|
|