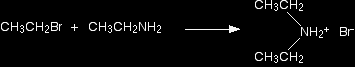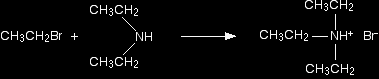MAKING AMINESThis page looks at the preparation of amines from halogenoalkanes (also known as haloalkanes or alkyl halides) and from nitriles. It only deals with amines where the functional group is not attached directly to a benzene ring. Aromatic amines such as phenylamine (aniline) are usually made differently and are discussed on a separate page. |
|
|
Note: Follow this link if you are mainly interested in the preparation of phenylamine. |
|
Making amines from halogenoalkanesThe halogenoalkane is heated with a concentrated solution of ammonia in ethanol. The reaction is carried out in a sealed tube. You couldn't heat this mixture under reflux, because the ammonia would simply escape up the condenser as a gas. We'll talk about the reaction using 1-bromoethane as a typical halogenoalkane. You get a mixture of amines formed together with their salts. The reactions happen one after another. Making a primary amine The reaction happens in two stages. In the first stage, a salt is formed - in this case, ethylammonium bromide. This is just like ammonium bromide, except that one of the hydrogens in the ammonium ion is replaced by an ethyl group.
There is then the possibility of a reversible reaction between this salt and excess ammonia in the mixture.
The ammonia removes a hydrogen ion from the ethylammonium ion to leave a primary amine - ethylamine. The more ammonia there is in the mixture, the more the forward reaction is favoured. |
|
|
Note: You will find considerable disagreement in textbooks and other sources about the exact nature of the products in this reaction. Some of the information you'll come across is simply wrong! You can read the arguments about the products of this reaction by following this link. Warning! That page is in the mechanism section of the site. Return to the current page using the BACK button on your browser. If you use the links at the bottom of that page, you could get seriously lost! |
|
Making a secondary amine The reaction doesn't stop at a primary amine. The ethylamine also reacts with bromoethane - in the same two stages as before. In the first stage, you get a salt formed - this time, diethylammonium bromide. Think of this as ammonium bromide with two hydrogens replaced by ethyl groups.
There is again the possibility of a reversible reaction between this salt and excess ammonia in the mixture.
The ammonia removes a hydrogen ion from the diethylammonium ion to leave a secondary amine - diethylamine. A secondary amine is one which has two alkyl groups attached to the nitrogen. Making a tertiary amine And still it doesn't stop! The diethylamine also reacts with bromoethane - in the same two stages as before. In the first stage, you get triethylammonium bromide.
There is again the possibility of a reversible reaction between this salt and excess ammonia in the mixture.
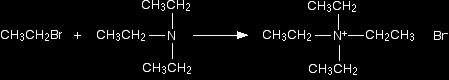
The ammonia removes a hydrogen ion from the triethylammonium ion to leave a tertiary amine - triethylamine. A tertiary amine is one which has three alkyl groups attached to the nitrogen. Making a quaternary ammonium salt The final stage! The triethylamine reacts with bromoethane to give tetraethylammonium bromide - a quaternary ammonium salt (one in which all four hydrogens have been replaced by alkyl groups).
This time there isn't any hydrogen left on the nitrogen to be removed. The reaction stops here. |
|
|
Note: This whole reaction sequence is a complete pain if you are going to have to learn it. It is much, much easier to work it out if you need to, provided you understand the mechanisms for the reactions. You can explore the mechanisms for the various stages of the reaction by following this link. This will lead you to several pages in the mechanism section of this site. If all you want to do is make some sense of the above reactions, it would probably pay you to just read the parts of those pages concerned with primary halogenoalkanes like bromoethane. |
|
What do you actually get if you react bromoethane with ammonia? Whatever you do, you get a mixture of all of the products (including the various amines and their salts) shown on this page. To get mainly the quaternary ammonium salt, you can use a large excess of bromoethane. If you look at the reactions going on, each one needs additional bromoethane. If you provide enough, then the chances are that the reaction will go to completion, given enough time. On the other hand, if you use a very large excess of ammonia, the chances are always greatest that a bromoethane molecule will hit an ammonia molecule rather than one of the amines being formed. That will help to prevent the formation of secondary (etc) amines - although it won't stop it entirely. Making primary amines from nitrilesNitriles are compounds containing the -CN group, and can be reduced in various ways. Two possible methods are described here. Reducing nitriles using LiAlH4 One possible reducing agent is lithium tetrahydridoaluminate(III) - often just called lithium tetrahydridoaluminate or lithium aluminium hydride. The nitrile reacts with the lithium tetrahydridoaluminate in solution in ethoxyethane (diethyl ether, or just "ether") followed by treatment of the product of that reaction with a dilute acid. Overall, the carbon-nitrogen triple bond is reduced to give a primary amine. For example, with ethanenitrile you get ethylamine:
Notice that this is a simplified equation - perfectly acceptable to UK A level examiners. [H] means "hydrogen from a reducing agent". |
|
Note: If you know about the reduction of aldehydes and ketones, you may know that they are also reduced by the similar compound NaBH4. However,
NaBH4 isn't a strong enough reducing agent to reduce nitriles. |
|
The reduction of nitriles using hydrogen and a metal catalyst The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts. Commonly quoted catalysts are palladium, platinum or nickel. The reaction will take place at a raised temperature and pressure. It is impossible to give exact details because it will vary from catalyst to catalyst. For example, ethanenitrile can be reduced to ethylamine by reaction with hydrogen in the presence of a palladium catalyst.
|
|
|
Note: Notice that this time the hydrogen is written normally as H2. This is a proper equation involving hydrogen gas - not a simplification. |
|
|
|