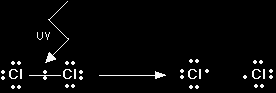EXPLAINING THE REACTION BETWEEN METHANE AND CHLORINE
A Free Radical Substitution ReactionThis page guides you through the mechanism for the substitution of one of the hydrogen atoms in methane by one chlorine atom. Multiple substitution is covered separately, and you will find a link at the bottom of the page. We are going to talk through this mechanism in a very detailed way so that you get a feel for what is going on. You couldn't possibly do the same thing in an exam. At the bottom of the page, you will find the condensed down version corresponding to the sort of answer you would produce in an exam. The role of the UV light The ultraviolet light is simply a source of energy, and is being used to break bonds. In fact, the energies in UV are exactly right to break the bonds in chlorine molecules to produce chlorine atoms.
|
|||||||||||||||||||||
|
Note: Only the outer electrons of the chlorine are shown. Notice also that it is quite acceptable to use a simple view of atomic structure. There is no point in using a complicated model of the atom if a simple one will do the job. |
|||||||||||||||||||||
Because we want to stress the fact that the chlorine atoms have single unpaired electrons, then we call them chlorine free radicals - or more usually just chlorine radicals. To show that a species (either an atom or a group of atoms) is a free radical, the symbol is written with a dot attached to show the unpaired electron. The splitting of the chlorine molecule would be shown as:
Free radicals are formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission. What happens to the chlorine radicals? There's nothing magic about reaction mechanisms. Reactions happen because things hit each other. If the conditions are right something useful might happen. In this case, you need to think about what the chlorine radicals are likely to hit, and what could happen as a result of that collision. At the moment the mixture contains
Let's start with the unproductive collisions. The least likely collision is between two chlorine radicals. There aren't very many of them in the mixture and so the chances of them hitting each other are relatively small. If they do collide, they will combine to form a chlorine molecule. That's worse than useless because it removes the active free radicals from the system.
A chlorine radical could also hit a chlorine molecule. If this happens there could possibly be an exchange of chlorine atoms, but nothing new would be formed. It is just a wasted collision.
|
|||||||||||||||||||||
|
Note: There is no difference between the chlorine atoms shown in bold type or ordinary type. They are shown differently so that the exchange is made clear. |
|||||||||||||||||||||
The productive collision happens if a chlorine radical hits a methane molecule.
The chlorine radical removes a hydrogen atom from the methane. That hydrogen atom only needs to bring one electron with it to form a new bond to the chlorine, and so one electron is left behind on the carbon atom. A new free radical is formed - this time a methyl radical, CH3
What happens to the methyl radicals? It depends what they collide with. There are three interesting collisions which need to be explored. Two of these involve a set-back to the reaction, and only one is useful. Luckily, the two unhelpful collisions don't happen very often, because they involve collisions between two free radicals - and there won't be many of these present in the mixture at any one time.
Even though the first reaction seems to produce what you want, the problem with both of these reactions is that they use up the free radicals in the system - we'll come back to that problem shortly. The second reaction, of course, also introduces an impurity into the mixture. So what is the useful collision? If a methyl radical hits a chlorine molecule (something that's quite likely to occur), the following change can happen:
The methyl radical takes one of the chlorine atoms to form chloromethane (which is what we want to make), but in the process generates another chlorine radical. This new chlorine radical can now go through the whole sequence again, and at the end will produce yet another chlorine radical - and so on and so on. The process is described as a free radical chain reaction. The chain continues because for every chlorine radical that goes in at the beginning, a new one is generated at the end. Chain termination Does this mean that one tiny burst of UV light, splitting one chlorine molecule into two free radicals, is enough to convert a whole reactions-worth of methane and chlorine into chloromethane and HCl? Sadly, no! As we've seen, there are collisions which result in the removal of free radicals without producing any new ones. These radicals can only be replaced by starting the process all over again with a new burst of light energy. In practice, then, the chains propagate many thousands of times, but eventually any chain will be brought to an end by one of these chain termination processes. Simplifying all this for exam purposes: The over-all process is known as free radical substitution, or as a free radical chain reaction. Chain initiation
Chain propagation reactions
Chain termination reactions
|
|||||||||||||||||||||