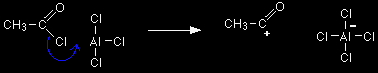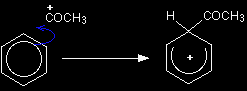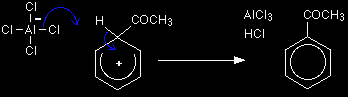EXPLAINING THE FRIEDEL-CRAFTS ACYLATION OF BENZENE
This page guides you through the mechanism for the Friedel-Crafts acyation of benzene involving an electrophilic substitution reaction between benzene and ethanoyl chloride in the presence of an aluminium chloride catalyst. |
|
|
Important! It would help if you first read the page What is electrophilic substitution? before you went on. |
|
The electrophilic substitution reaction between benzene and ethanoyl chlorideThe formation of the electrophile If you are going to replace a hydrogen atom in a benzene ring by CH3CO, then the electrophile must be the ion CH3CO+. The positive charge must be on the carbon atom, because that's what gets attached to the ring. |
|
|
Note: If you don't understand why the electrophile has got to be CH3CO+, then you really should look at What is electrophilic substitution? before you go on. If you are going to substitute X onto the ring, then the electrophile must be X+. If you are going to insert an CH3CO group onto the ring, then the electrophile must be CH3CO+. |
|
Aluminium chloride, AlCl3, is an electron deficient molecule. It is covalently bonded, but because the aluminium is only forming 3 bonds, and has no lone pairs, there are only 6 electrons around the aluminium atom rather than 8. It takes a chlorine (as a chloride ion) from the ethanoyl chloride, and forms a co-ordinate (dative covalent) bond with it.
|
|
|
Help! A co-ordinate bond is a covalent bond in which both electrons originally came from the same atom. In this case, both the electrons in the bond come from the chlorine being removed from the ethanoyl chloride. |
|
The equation simplified
The electrophilic substitution mechanism Stage one As the CH3CO+ ion approaches the delocalised electrons in the benzene, those electrons are strongly attracted towards the positive charge. Two electrons from the delocalised system are used to form a new bond with the CH3CO+ ion. Because those two electrons aren't a part of the delocalised system any longer, the delocalisation is partly broken, and in the process the ring gains a positive charge.
|
|
|
Help! Don't be confused that we have reversed the way we have written the CH3CO+ ion to show it as +COCH3. This is just so that it is easier to draw the mechanism tidily in the same way that the general mechanism was drawn on the page What is electrophilic substitution? |
|
The hydrogen shown on the ring is the one which was already attached to that top carbon atom. We need to show it there because it has to be removed in the second stage. Stage two The second stage involves the AlCl4-, which was produced at the same time as the CH3CO+ ion.
One of the aluminium-chlorine bonds breaks and both electrons from it are used to join to the hydrogen. This removes the hydrogen from the ring to form HCl, and re-generates the aluminium chloride catalyst in the process. The electrons which originally joined the hydrogen to the ring are now used to re-establish the delocalised system.
To menu of electrophilic substitution reactions. . . |
|