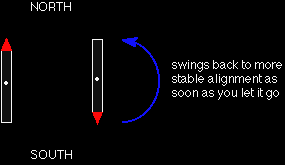
WHAT IS NUCLEAR MAGNETIC RESONANCE (NMR)?This page describes what a proton NMR spectrum is and how it tells you useful things about the hydrogen atoms in organic molecules. The background to NMR spectroscopyNuclear magnetic resonance is concerned with the magnetic properties of certain nuclei. On this page we are focussing on the magnetic behaviour of hydrogen nuclei - hence the term proton NMR or 1H-NMR. Hydrogen atoms as little magnets If you have a compass needle, it normally lines up with the Earth's magnetic field with the north-seeking end pointing north. Provided it isn't sealed in some sort of container, you could twist the needle around with your fingers so that it pointed south - lining it up opposed to the Earth's magnetic field. It is very unstable opposed to the Earth's field, and as soon as you let it go again, it will flip back to its more stable state.
Hydrogen nuclei also behave as little magnets and a hydrogen nucleus can also be aligned with an external magnetic field or opposed to it. Again, the alignment where it is opposed to the field is less stable (at a higher energy). It is possible to make it flip from the more stable alignment to the less stable one by supplying exactly the right amount of energy.
The energy needed to make this flip depends on the strength of the external magnetic field used, but is usually in the range of energies found in radio waves - at frequencies of about 60 - 100 MHz. (BBC Radio 4 is found between 92 - 95 MHz!) It's possible to detect this interaction between the radio waves of just the right frequency and the proton as it flips from one orientation to the other as a peak on a graph. This flipping of the proton from one magnetic alignment to the other by the radio waves is known as the resonance condition. The importance of the hydrogen atom's environment What we've said so far would apply to an isolated proton, but real protons have other things around them - especially electrons. The effect of the electrons is to cut down the size of the external magnetic field felt by the hydrogen nucleus.
Suppose you were using a radio frequency of 90 MHz, and you adjusted the size of the magnetic field so that an isolated proton was in the resonance condition. If you replaced the isolated proton with one that was attached to something, it wouldn't be feeling the full effect of the external field any more and so would stop resonating (flipping from one magnetic alignment to the other). The resonance condition depends on having exactly the right combination of external magnetic field and radio frequency. How would you bring it back into the resonance condition again? You would have to increase the external magnetic field slightly to compensate for the effect of the electrons. Now suppose that you attached the hydrogen to something more electronegative. The electrons in the bond would be further away from the hydrogen nucleus, and so would have less effect on the magnetic field around the hydrogen.
|
|
|
Note: Electronegativity is a measure of the ability of an atom to attract a bonding pair of electrons. If you aren't happy about electronegativity, you could follow this link at some point in the future, but it probably isn't worth doing it now! |
|
The external magnetic field needed to bring the hydrogen into resonance will be smaller if it is attached to a more electronegative element, because the hydrogen nucleus feels more of the field. Even small differences in the electronegativities of the attached atom or groups of atoms will make a difference to the magnetic field needed to achieve resonance. Summary For a given radio frequency (say, 90 MHz) each hydrogen atom will need a slightly different magnetic field applied to it to bring it into the resonance condition depending on what exactly it is attached to - in other words the magnetic field needed is a useful guide to the hydrogen atom's environment in the molecule. Features of an NMR spectrumA simple NMR spectrum looks like this:
|
|
|
Note: The nmr spectra on this page have been produced from graphs taken from the Spectral Data Base System for Organic Compounds (SDBS) at the National Institute of Materials and Chemical Research in Japan. It is possible that small errors may have been introduced during the process of converting them for use on this site, but these won't affect the argument in any way. |
|
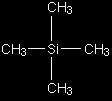
The peaks There are two peaks because there are two different environments for the hydrogens - in the CH3 group and attached to the oxygen in the COOH group. They are in different places in the spectrum because they need slightly different external magnetic fields to bring them in to resonance at a particular radio frequency. The sizes of the two peaks gives important information about the numbers of hydrogen atoms in each environment. It isn't the height of the peaks that matters, but the ratio of the areas under the peaks. If you could measure the areas under the peaks in the diagram above, you would find that they were in the ratio of 3 (for the larger peak) to 1 (for the smaller one). That shows a ratio of 3:1 in the number of hydrogen atoms in the two environments - which is exactly what you would expect for CH3COOH. The need for a standard for comparison - TMS Before we can explain what the horizontal scale means, we need to explain the fact that it has a zero point - at the right-hand end of the scale. The zero is where you would find a peak due to the hydrogen atoms in tetramethylsilane - usually called TMS. Everything else is compared with this.
You will find that some NMR spectra show the peak due to TMS (at zero), and others leave it out. Essentially, if you have to analyse a spectrum which has a peak at zero, you can ignore it because that's the TMS peak. TMS is chosen as the standard for several reasons. The most important are:
The chemical shift The horizontal scale is shown as A peak at a chemical shift of, say, 2.0 means that the hydrogen atoms which caused that peak need a magnetic field two millionths less than the field needed by TMS to produce resonance. A peak at a chemical shift of 2.0 is said to be downfield of TMS. The further to the left a peak is, the more downfield it is. Solvents for NMR spectroscopy NMR spectra are usually measured using solutions of the substance being investigated. It is important that the solvent itself doesn't contain any simple hydrogen atoms, because they would produce confusing peaks in the spectrum. There are two ways of avoiding this. You can use a solvent such as tetrachloromethane, CCl4, which doesn't contain any hydrogen, or you can use a solvent in which any ordinary hydrogen atoms are replaced by its isotope, deuterium - for example, CDCl3 instead of CHCl3. All the NMR spectra used on this site involve CDCl3 as the solvent. Deuterium atoms have sufficiently different magnetic properties from ordinary hydrogen that they don't produce peaks in the area of the spectrum that we are looking at. |
|
|
Note: Several text books say that deuterium atoms don't have a magnetic field. It isn't true - they do have a field but it is less than an ordinary hydrogen atom. |
|
|
|