Reversible reactions
Reversible reactions are ones that can go forward and backwards depending on the conditions. A very simple example is melting ice, this is not a reaction, yet it has the same idea. If you heat a block of ice it melts into water, consider this the forward direction of the reaction. If you freeze the water, it turns back to ice, this is the backward reaction. This change depends on temperature.
Dehydration and Hydration:
Assume we have a hydrated salt, copper sulphate for example. If you heat the salt you get two products. They are water and anhydrous copper sulphate. This is a reversible reaction because if you cool the mixture of the products again, you get hydrated copper sulphate back. Note: hydrated copper sulphate is blue crystals. Anhydrous copper sulphate is white powder but it forms a blue solution with water.
| CuSO4 ⇋ CuSO4 + 5H2O | |
| →Heating→ | ←Cooling← |
Equilibrium:
Some reversible reactions are very unique, at a certain point, the reaction will be going forward and backwards at the same time and at the same rate. This is called the state of equilibrium. In the state of equilibrium, the rate of forward reaction is equal to the rate of backward reaction and the amount of products and reactants remain constant.
Reactants ⇋ Products
In the state of equilibrium, conditions could be changed and factors could be altered to increase the rate of the forward reaction and thus increasing the yield of the products (Shifting the equilibrium to the right) or to increase the rate of the backward reaction and thus obtaining the reactants (Shifting the equilibrium to the left).
For example, if in a reversible reaction the forward reaction is exothermic, that means that making the product increases the temperature. If we want more products, we will decrease the temperature, the equilibrium will be shifted forward and the rate of the forward reaction will increase to try and raise the temperature back to normal. This will increase the yield of the product. The rate of the backward reaction is endothermic. So if we want the reactants and not the products, we will increase the temperature. This will shift the equilibrium to the left the rate of the backward reaction will increase and the rate of the forward reaction will decrease, thus absorbing the temperature which drops back to normal and the amount of reactants increase.
If the reactants are solids or liquids and one or more of the products are a gas, adjusting the pressure will have an effect on the equilibrium. This is because the gas of the products exerts pressure. If want more of the product, we will decrease the pressure. This shifts the equilibrium to the right and increases the rate of the forward reaction making more gas, thus raising the pressure back to normal. This increases the yield of the products. If we want more of the reactants instead, we could increase the pressure, this will shift the equilibrium to the left and increase the rate of backward reaction. This is to convert some of the gas which exerts pressure to reactants to drop the pressure down to the normal. This gives us more of the reactants. If the case is opposite (the gas is in the reactants rather than the products) the opposite is done.
It is all about understanding the characteristics of the forward and the backward reaction. If the forward reaction increases temperature, you could drop the temperature to keep it moving forward (shifting the equilibrium to the right) to obtain more products. If you want less product and more reactants, you could increase the temperature to keep it moving backwards (shifting the equilibrium to the left) and obtain more products. If the forward reaction increases pressure (a gas is in the products) then you could decrease the temperature to keep it moving forward (shifting the equilibrium to the right) to obtain more products. If you want less product and more reactants, you could increase pressure to keep it moving backwards (shifting the equilibrium to the left) to obtain more reactant. In other words, see what the forward reaction does and do the opposite to keep it moving forward. Or see what the backward reaction does and do the opposite to make it move backward.
Haber process:
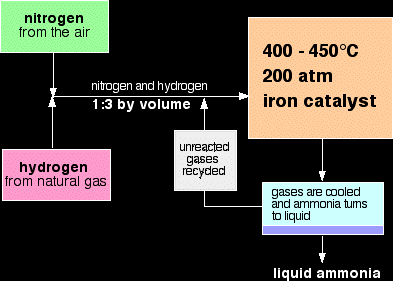
Haber process is the manufacture of ammonia by reacting nitrogen and hydrogen together. Nitrogen needed for this process is obtained from fractional distillation of liquid air. Hydrogen needed could be obtained by three ways, either reacting methane with steam, electrolysis of brine or cracking of alkanes.
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g)
| →→→→→→→→→→ | ←←←←←←←←←← |
|---|---|
|
|
How to obtain more ammonia:
In this reaction, very little of the reactants react forming ammonia. Some factors must be changed to shift the equilibrium to the right and obtain more ammonia. We want to shift the equilibrium to the right, so we should do the opposite of what the forward reaction does:
- The forward reaction is exothermic. We should decrease the temperature. This reaction should be done at high temperature; we want to lower this temperature. But beware that a very low temperature will make the reaction very slow since particles will lose their kinetic energy. A temperature which is low enough to shift the equilibrium to the left and isn’t too low to make the process slow is 450ºC.
- The forward reaction decreases the pressure. This is because we’ve got 4 moles of gases (1 from nitrogen and 3 from hydrogen); each mole needs a volume of 24dm3 which means that the reactants need a volume of 96dm3. In the products we have only 2 moles of gas which come from ammonia gas. This means it needs 48dm3. The reactants exert more pressure than the products. We should increase the pressure. This will make the equilibrium shift to the right to convert more reactants into products to decrease the high pressure and drop it down to normal. The optimum pressure for this reaction is 200atm. Increasing the pressure also increases the rate since it increases the frequency of effective collisions between the particles.
- To speed the reaction even more, a catalyst could be used. The best catalyst for this reaction is powdered iron. A catalyst increases the rate of both the forward and the backward reaction, but since we’ve changed other factors, the equilibrium will still be shifted to the right. A catalyst does not have an effect on the position of the equilibrium.
Changing the Concentration of Reactants:
To make sure that all the reactants react and form ammonia, a trick could be used. The boiling point of ammonia is -33ºC, nitrogen is -196ºC and hydrogen is 299ºC. When equilibrium is reached, there will be a mixture of gases (ammonia, nitrogen & hydrogen). This mixture is cooled to about -40ºC so that ammonia condenses but nitrogen and hydrogen don’t. Ammonia is collected and stored. The unreacted remainder of the reactants is then recycled and the ideal conditions are brought back in for it to react. This is repeated until no more of the reactants remain.
Uses of Ammonia:
- Manufacture of fertilisers
- Manufacturing of nitric acid