Rates of Reaction
The rate of a reaction can be measured by the rate at which a reactant is used up, or the rate at which a product is formed.
The temperature, concentration, pressure of reacting gases, surface area of reacting solids, and the use of catalysts, are all factors which affect the rate of a reaction.
Chemical reactions can only happen if reactant particles collide with enough energy. The more frequently particles collide, and the greater the proportion of collisions with enough energy, the greater the rate of reaction.
Measuring rates of reaction
There are two ways to find the rate of a reaction:
- Measure the rate at which a reactant is used up,
- Measure the rate at which a product is formed.
The method chosen depends on the reaction being studied. Sometimes it is easier to measure the change in the amount of a reactant that has been used up; sometimes it is easier to measure the change in the amount of a product that has been produced.
Things to measure:
The measurement itself depends on the nature of the reactant or product:
- The mass of a substance - solid, liquid or gas - is measured with a balance
- The volume of a gas is usually measured with a gas syringe, or sometimes an upside-down measuring cylinder or burette
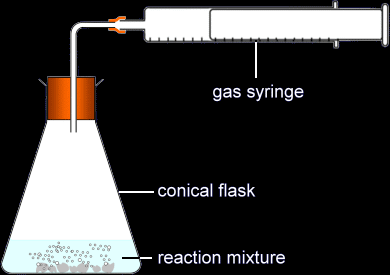
Measuring the production of a gas using a gas syringe
It is usual to record the mass or total volume at regular intervals and plot a graph. The readings go on the vertical axis, and the time goes on the horizontal axis.
![]()
For example, if 24cm3 of hydrogen gas is produced in two minutes, the mean rate of reaction = 24 ÷ 2 = 12cm3 hydrogen / min.
Factors affecting the rate
You will be expected to remember the factors that affect the rate of reactions, and to plot or interpret graphs from rate experiments.
How to increase the rate of a reaction:
The rate of a reaction increases if:
- The temperature is increased,
- The concentration of a dissolved reactant is increased,
- The pressure of a reacting gas is increased,
- Solid reactants are broken into smaller pieces,
- A catalyst is used.
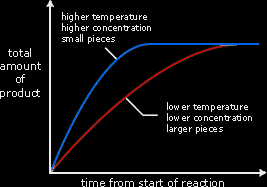
The graph above summarises the differences in the rate of reaction at different temperatures, concentrations and size of pieces. The steeper the line, the greater the rate of reaction. Reactions are usually fastest at the beginning, when the concentration of reactants is greatest. When the line becomes horizontal, the reaction has stopped.
Collisions and reactions
You will be expected to explain, in terms of particles and their collisions, why changing the conditions of a reaction changes its rate.
Collisions:
For a chemical reaction to occur, the reactant particles must collide. Collisions with too little energy do not produce a reaction.
The collision must have enough energy for the particles to react. The minimum energy needed for particles to react is called the activation energy.
Changing concentration or pressure:
If the concentration of a dissolved reactant is increased, or the pressure of a reacting gas is increased:
- There are more reactant particles in the same volume
- There is a greater chance of the particles colliding
- The rate of reaction increases
Changing particle size:
If a solid reactant is broken into small pieces or ground into a powder:
- Its surface area is increased
- More particles are exposed to the other reactant
- There is a greater chance of the particles colliding
- The rate of reaction increases
Changing the temperature:
If the temperature is increased:
- The reactant particles move more quickly
- More particles have the activation energy or greater
- The particles collide more often, and more of the collisions result in a reaction
- The rate of reaction increases
Using a catalyst:
Catalysts increase the rate of reaction without being used up. They do this by lowering the activation energy needed. With a catalyst, more collisions result in a reaction, so the rate of reaction increases. Different reactions need different catalysts.
Catalysts are important in industry because they reduce costs.