Metals
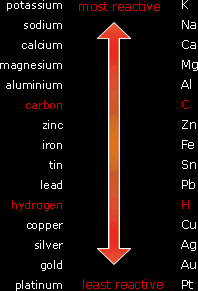
The Reactivity Series of Metals:The reactivity series is metal is an arrangement of the metals (and carbon and hydrogen) in order of their reactivity starting with the most reactive metal at the top and ending with the least reactive metal at the bottom. The reactivity of a metal is determined by its ability to form a positive ion. For example, potassium is extremely reactive because it has only one valence electron, so it is very easy to lose it forming a positive ion. One the other hand, copper is a weakly reactive metal because it has more valence electrons so it is harder for it to become a positive ion. Reactions of Metals:The reactivity series of metals was deduced by performing several experiments in the lab which enabled scientists to arrange metals according to their reactivity with dilute acid, oxygen (air), and water. |
 |
Reactions with Dilute Hydrochloric Acid:
In the previous chapter, you studied those reactions involving a metal and an acid are used to prepare soluble salts and that this method is only suitable for preparing salts of moderately reactive metals (MAZIT). This is because any metal more reactive that magnesium will react very violently with acids which is dangerous.
Metal + HCl → Metal Chloride + Hydrogen
The photo on the right shows magnesium reacting with dilute hydrochloric acid. Those effervescences are caused by the evolution of hydrogen gas, which is a product in this reaction. This reaction was repeated using the other metals of the reactivity series. The rate of evolution of hydrogen gas in each experiment was measured. The metals were arranged in order of reactivity starting with the most reactive metal which had the highest rate of effervescence of hydrogen gas. The rate of effervescence is also the rate of this reaction is measured by measuring the volume of hydrogen produced per unit time.
|
 |
Reactions with Oxygen in Air:
Most metals react with oxygen from air forming a metal oxide. You have previously studied that metal oxides are basic oxides and that some of them are insoluble in water and some of them are soluble in water forming an alkaline solution. The most reactive metals like potassium, sodium, calcium and magnesium react with oxygen with a very bright flame and producing white ashes and their oxides are soluble. Moderately reactive metals like aluminum and zinc react with oxygen forming white powdered ashes but their oxides are insoluble. Iron and copper react very slowly with oxygen. The result of iron oxygen reactions is rust which is reddish brown iron oxide. When a copper lump reacts with oxygen, a white layer of black copper oxide forms on it. When the lump gets covered by this layer; the reaction stops. Oxides of iron and copper are insoluble. Metals that are less reactive than copper like silver, gold and platinum do not react with oxygen.
Note: When aluminum reacts with oxygen, a layer of aluminum oxide adheres and covers the aluminum. At this point no further reaction can take place.
Reactions of Metals with Water and Steam:
Some metals are so reactive that they will just react with water immediately if they come in contact. Other metals will react slowly will cold water, but with steam they react much faster. And other metals can only react slowly with steam. Unreactive metals such as silver and gold do not react with water.
Potassium, sodium and calcium react vigorously with cold water and may catch on fire. The products of these reactions are metal hydroxide and hydrogen gas. If hydrogen gas being produced accumulates it may ignite and cause an explosion.
Metal + Water → Metal hydroxide + Hydrogen
E.g.: 2Na + 2H2O → 2NaOH + H2
Magnesium, aluminum, zinc and iron are less reactive. They react with steam forming metal oxide and hydrogen. Magnesium and aluminum will react vigorously with steam while zinc and iron react slowly.
Metal + Steam → Metal Oxide + Hydrogen
E.g.: Magnesium + Steam → Magnesium oxide + Hydrogen
Competition Reactions in Solid State:
Previously you’ve studied displacement reactions which are pre-formed in aqueous states. A very similar reaction takes place in the solid state, it is called thermite reaction. This reaction is used to repair damaged railway lines. In this reaction, aluminum and iron (III) oxide are the reactants. In the reaction, aluminum removes the oxygen ion from iron and bonds with it. This happens because aluminum is more reactive than iron. The products are aluminum oxide and iron in molten form. In the fixing procedure, the reactants are put in the cut in the railway line and the reaction is triggered by heating using a magnesium fuse. The reaction leaves aluminum oxide and molten iron with then condenses in the cut welding it. Like displacement reactions, this reaction is exothermic.
2Al + Fe2O3 → Al2O3 +2Fe
Competition Reactions in Aqueous State:
These are ordinary displacement reactions in which the two positive ions compete for the negative ion. The ion of the more reactive metal wins. Zinc is higher than copper in the reactivity series. If zinc is added to a solution of copper nitrate, a displacement reaction will take place in which the zinc will displace the copper ion from the solution in its salt. The products of this reaction are zinc nitrate and copper. Copper salt solutions have a blue color which fades away as the reaction proceeds because the concentration of the copper salt decreases. This type of reaction also helped in confirming reactivity of metals since the more reactive metal displaces the less reactive one.
Zn + Cu(NO3)2 → Zn(NO3)2 + Cu
Action of Heat on Metal Compounds:
Applying heat to a metal compound such as potassium nitrate will cause it to decompose into potassium nitrite and oxygen. This is a thermal decomposition reaction.
| Metal: | Anion: | ||
| Nitrate (NO3) | Carbonate (CO3) | Hydroxide (OH) | |
| Potassium Sodium |
Metal Nitrate → Metal nitrite + Oxygen | NO DECOMPOSITION | |
| Calcium Magnesium Aluminum Zinc Iron Lead Copper |
Metal Nitrate → Metal oxide + Nitrogen dioxide + Oxygen | Metal Carbonate → Metal oxide + Carbon dioxide | Metal hydroxide →Metal oxide + Hydrogen |
| Silver Gold |
Metal Nitrate → Metal + Nitrogen dioxide + Oxygen | Metal Carbonate → Metal + Carbon dioxide + Oxygen | - |
Silver and gold hydroxides do not exist.
Ions of more reactive metals tend to hold on tightly to their anions and do not decompose easily this is why lots of heat is needed.
Extracting Metals From Their Ores:
Most metals do not exist in nature as pure elements. Instead, they are found as naturally occurring compounds called ores. Ores are naturally occurring minerals from which a metal can be extracted. Most ores are metals oxide, carbonate or sulfide mixed with other impurities. The extraction of metal from ores begun long ago when people started purifying iron from its iron oxide ore by reducing it using charcoal. This was possible because carbon is more reactive than iron so it can reduce it take the oxygen ion from it. But then other metals were discovered which were higher than carbon in the reactivity series. Those metals were not possibly extracted from their ores until in the 19th century when a method of extracting them by electrolysis was invented. The method extracting a metal depends on its reactivity.
| Metals - in decreasing order of reactivity | Reactivity |
|---|---|
| Extract by electrolysis | |
| Extract by reaction with carbon or carbon monoxide | |
| Extracted by various chemical reactions |
Extraction of Aluminum:
Aluminum exists naturally as aluminum oxide (alumina) in its ore, which is called bauxite. Because aluminum is a very reactive metal, it holds on very tightly to the anion it bonds with, which is oxide in this case. This is why the best way to extract and purify aluminum is by electrolysis in a cell like the one below.
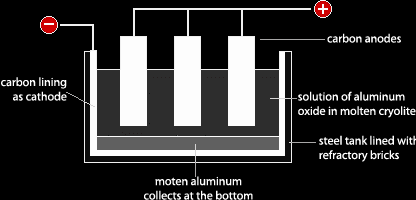
In this cell, the electrodes are made of graphite (Carbon). The cathode is a layer at the bottom of the cell and the anodes are bars dipped in the electrolyte. The electrolyte in this process is a molten mixture of aluminum oxide and cryolite. Aluminum oxide by its self has a very high melting point of 2050oC which is higher than the melting point of the steel container in which this process is done. That means the steel container will melt before the aluminum oxide. This is why aluminum oxide is mixed with cryolite which decreases the melting point of it to under 1000oC, thus saving a lot of money because heating is expensive and preventing the steel container from melting. Heat must be continuously supplied to the mixture to keep it molten. Aluminum oxide does not conduct electricity when solid because it does not have free mobile ions to carry the charge.
- Aluminum oxide is purified from impurities of oxide by adding sodium hydroxide
- Aluminum oxide is mixed with cryolite and put in the electrolysis cell
- Heat is given in until the mixture becomes molten
- Electrolysis start
- Oxide ions get attracted to the anode and discharged (oxidation); 2O2-, 4e → O2
- Aluminum ions get attracted to the cathode and discharged and settle at the bottom
of the container (reduction); Al3+ + 3e → Al - Oxygen gas evolves and is collected with waste gases
- Aluminum is sucked out of the container at regular intervals
Oxygen gas which evolves reacts with carbon from the cathode forming CO2. The cathode gets worn away. To solve this, the cathode is replaced at regular intervals. Heat supply is very expensive; this is why cryolite is used to decrease the melting point of aluminum oxide and this process is done in plants which use hydroelectric energy because it is cheap.
Uses of aluminum:
- Construction of air-craft bodies because aluminum is very strong and very light and it is resistant to corrosion
- Food containers because it is resistant to corrosion
- Overhead power cables because it conducts electricity, is very light, malleable and ductile. Although it is strengthened with steel core
Extraction of Iron:
The ore of iron is called hematite. It consists of 60% iron in form of Iron oxide (Fe2O3) with other impurities such as silicon
oxide (SiO2). This process takes place in a tower called a Blast furnace.
| Substances | Products and Waste Materials |
- Substances are put in the blast furnace
- The process starts by blowing in hot air at the bottom of the furnace
- Coke burns in oxygen from the hot air producing carbon dioxide; C + O2 → CO2
- Heat makes lime stone decompose into calcium oxide and carbon dioxide; CaCO3 → CaO + CO2
- Carbon dioxide produced goes up the furnace and reacts with more coke up there producing
carbon monoxide; CO2 + C → 2CO - Carbon monoxide is a reducing agent. It rises further up the furnace where it meets iron oxide and starts reducing it producing iron and carbon dioxide; Fe2O3 + 3CO → 2Fe + 3CO2
- Calcium oxide which was produced from the thermal decomposition of lime stone is a base. It reacts with impurities of hematite such as silicon oxide which is acidic forming calcium silicate which is called slag; CaO + SiO2 → CaSiO3
- Molten Iron and slag produced trickles down and settles at the bottom of the furnace. Iron is denser than slag so it settles beneath it.
Iron and slag are tapped off separately at regular intervals and pure iron is collected alone - Waste gases such as carbon dioxide formed in the process and nitrogen and other gases from air blown in escape at the top of the furnace.
Conversion of Iron into Steel:
Iron produced in the blast furnace is called pig iron. It contains 4% carbon as well as other impurities such as sulfur, silicon and phosphorus which make it hard and brittle. It got that name from the fact that it has to be poured into mould called pigs before it is converted into steel. Most of produced iron is converted into steel because steel has better properties.
Making steel out of pig iron is a process done in a basic oxygen furnace:
- Molten pig iron is poured into the oxygen furnace
- A water cooled lance is introduced which blows oxygen onto the surface of the molten iron
- Impurities start to react
- Carbon is oxidized into carbon monoxide and carbon dioxide and escape
- Sulfur is oxidized into sulfur dioxide and escapes
- Silicon and phosphorus are oxidized into silicon oxide and phosphorus pentoxide which are solids.
- Calcium oxide (lime) is added to remove the solid impurities as slag which is skimmed off the surface
- Throughout the process, sample of the iron are being taken and analyzed for the percentage of carbon present in it. When the percentage of carbon desired is reached, the furnace is switched off and the steel is collected.
There are many different forms of steel. Each has different components and properties and is used for different purposes.
| Steel | Composition | Properties | Uses |
| Mild Steel | 99.5% Iron 0.5% Carbon |
Easily worked lost brittleness | Car bodies large structures Machinery |
| Hard Steel | 99% Iron 1% Carbon |
Tough and brittle | Cutting tools and chisels |
| Stainless Steel | 87% Iron 13% Manganese |
Tough and springy | Drill bits and springs and chemical plants |
| Manganese Steel | 74% Iron 18% Chromium 8% Nickel |
Tough and resistant to corrosion | Cutlery and surgical tools, kitchen sinks |
| Tungsten Steel | 95% Iron 5% Tungsten |
Tough and hard even at high temperatures | Edges of high speed cutting tools |
Extraction of Zinc:
The ore of zinc is called zinc blende and it is made of zinc sulfide. Zinc is obtained from zinc sulfide by converting it into zinc oxide then reducing it using coke, but first zinc sulfide must be concentrated.
Zinc sulfide from zinc blende is concentrated by a process called froth floatation. In this process, the ore is crushed and put into tanks of water containing a frothing agent which makes the mixture froth up. Hot air is blown in and froth starts to form. Rock impurities in the ore get soaked and sink to the bottom of the tank. Zinc sulfide particles cannot be soaked by water; they are lifted by the bubbles of air up with the froth and are then skimmed off. This is now concentrated zinc sulfide.
Then, zinc sulfide gets heated very strongly with hot air in a furnace. Zinc sulfide reacts with oxygen from the air to produce zinc oxide and sulfur dioxide gas which escapes as waste gas.
2ZnS + 3O2 → 2ZnO + 2SO2
Sulfur dioxide is used in the manufacture of sulfuric acid.
Zinc oxide produced is put into a furnace with powdered coke. The mixture is heated till 1400oC. Carbon from the coke reduces the zinc oxide into zinc producing carbon monoxide which escapes as waste gas.
ZnO + C → Zn + CO
Carbon monoxide produced is hot and is used to heat the furnace to reduce heating costs. The pure zinc produced is collected and left to cool down. Zinc is used in many ways like the production of the alloy brass, galvanization and making car batteries.
Extraction of Copper:
Copper is one of the most popular metals. Native copper occurs in some regions in the world. Otherwise, copper exists in its ore, copper pyrites (2CuFeS2). You have studied before that copper can be purified by electrolysis. It can also be extracted from it ore by converting pyrites into copper sulfide by reacting it with oxygen:
2CuFeS2 + 4O2 → Cu2S + 3SO2 + 2FeO
Sulfur oxide produced escapes as waste gas and iron oxide impurities are removed by heating the mixture with silicon converting it in to iron silicate which is run off. The remaining copper sulfide is then heated strongly with air. Copper sulfide reacts with oxygen from air producing sulfur oxide which escapes as waste gas and pure copper.
Cu2S + O2 → 2Cu + SO2
Thus copper is extracted.
Uses of Copper:
- In electrical wires because it is a perfect electrical conductor and very ductile, malleable and cheap
- Making alloys such as bronze and brass
- Cooking utensils because it conducts heat and it is has high melting and boiling points and also resists corrosion
- Electrodes because it is a good conductor of electricity
- Water pipes because it is resistant to corrosion