Lab Skills and Separating Methods
This unit deals with lab equipment and methods of separating solutions.
Element, Compounds and Mixtures:
Speaking about the chemistry of matter, we have only 3 types of matter. These are elements, mixtures and compounds. Long ago, scientists found out that the smallest unit of a matter is called an atom. An element is extremely pure because it is made up of only one type of atoms. For example a pure gold ring has only the element Gold (Au) in it. Compounds are very pure too, a compound is made up of one type of a particle called molecule. A molecule consists of two or more atoms chemically bonded together. Carbon Dioxide (CO2) gas is a compound. A mixture however is not pure at all. A mixture is just two or more elements or compounds mixed together, but not chemically bonded. For example if you dissolve some table salt, which is a compound called sodium chloride (NaCl) in some water, which is also another compound (H2O), you will get a mixture of Sodium Chloride in water, but there are absolutely no bonds between the Sodium Chloride molecules and water molecules. Air is another good example of mixtures. Air is just a mixture of gases floating around each other like Nitrogen and Oxygen, which are pure elements. Air also contains compounds such is Carbon Dioxide.
 |
 |
 |
Iron: lustrous metallic |
Pure Calcium Carbonate |
Sea water is a mixture of Salts and Water |
Elements:
Elements are substances that consist of only one kind of atoms and cannot be broken down into simpler substances by chemical means.
Can you recognise elements, compounds and mixtures?
- An element contains just one type of atom.
- A compound contains two or more types of atom joined together.
- A mixture contains two or more different substances that are not joined together.
- The different substances in a mixture can be elements or compounds.
Diatomic Molecules are molecules made of two atoms of the same element, such as Chlorine molecules (Cl2) and Oxygen molecules (O2).
Since particles in mixtures have no chemical bonding between them, they could be easily separated by physical means. The method of separation however depends on the type of the mixture, and some of the physical properties of its components.
We have 4 types of mixtures:
- Solid/Solid mixtures
- Solid/liquid mixtures
- Liquid/liquid mixtures
- Gas/gas mixtures
Separating Solid/Solid Mixtures:
By Magnet:
This method is used to separate a mixture of two solids. One condition must be present though. This is that one of the solids is magnetic. For example if we have a mixture of sand and iron chips. We can separate them by:
- Pouring the mixture in a dish,
- Introducing a magnet just above the mixture.
 |
→ |  |
The iron chips will immediately get attracted to the magnet leaving sand behind.
By Sublimation:If we have a mixture of two solids, one of them undergoes sublimation we can easily separate them by heating the mixture using a Bunsen burner. One solid might melt while the other one will directly sublime into a gas. This process must be done in a fume cupboard in order to collect the gas. |
 |
By Solvent Extraction Method:
This method is used one of the solids is water soluble, while the other is insoluble, for example a sand and salt mixture. In this method, the mixture is put in a beaker and water is added to it. The mixture is stirred on gentle heating to make the salt dissolve in the water quickly. Then the mixture is filtered using a filter funnel and filter paper. The residue will be the insoluble sand and the filtrate will be the salt solution. The sand is dried and collected. The salt is obtained from the solution by either the evaporation or the crystallisation method which will be studied later on.
Separating Solid/Liquid Mixtures:
Solubility:
A solution is formed when a solute is dissolved in a solvent.
Solute: This is a substance that dissolves in a solvent forming a solution
Solvent: This is a substance in which a solute dissolves forming a solution
Solution: A uniform mixture which is formed when a solute is dissolved in a solvent.
Dilute Solution: A solution with a small amount of solute/dm3.
Concentrated Solution: A solution with large amounts of solute/dm3.
Concentration: The amount of solute (in grams or moles) that can dissolve in 1dm3 of a solvent.
Saturated Solution: A very concentrated solution with the maximum amount of solute that dissolves in it already dissolved in it.
If you leave a hot saturated solution to cool, crystals of the solute will form. This is because as the temperature decreases the solvent can hold less solute so excess will form in the form of crystals.
The rate of dissolving can be increased by:
- Increasing temperature,
- More stirring,
- Crushed solute (larger surface area).
Solubility: The maximum amount of solute that can dissolve in 100g of water at a particular temperature.
If we want to find the solubility of table salt (sodium chloride) at 30oC, we do the follow these steps:
- Use a balance to measure 100g of water accurately,
- Pour the 100g of water into a beaker,
- Heat the water to 30ºC using a Bunsen burner and a thermometer,
- Using a spatula, add a considerable mass of the table salt into the water and stir,
- If the mass of salt dissolves completely, add the same amount again and stir, repeat this if the mass keeps dissolving completely until you start seeing excess of the salt not dissolving at the bottom of the beaker,
- You have to record the masses of salt you are adding each time and when you start seeing the excess stop adding salt and sum up the amount of salt you added. Call this Mass1,
- Filter the solution. The excess of salt will be the residue, dry it and weigh it. Call this Mass2,
- The amount of table salt that was dissolved in water is Mass1 - Mass2,
- This is the solubility of table salt at 30ºC.
Solubility increases as temperature increases. This is because the intermolecular spaces between the water molecules increase with temperature, giving more space for the solute’s molecules.
By Evaporation (For Soluble Solid/Liquid Solutions):
- Put solution in a beaker,
- Set the apparatus (Tripod with a gauze above it and a Bunsen burner below it),
- Put the beaker on the gauze,
- Start heating the solution slowly.
The liquid will evaporate completely leaving the solute behind in powder form.
By Crystallization (For Soluble Solid/Liquid Solutions):
- Put solution in a beaker,
- Set the apparatus (Tripod with a gauze above it and a Bunsen burner below it),
- Insert a glass rod in the beaker,
- Turn on the Bunsen burner and continuously dip the glass rod in the solution,
- When you see crystals of the solute starting to form on the glass rod, turn of
the Bunsen burner. (This is crystallization point), - Leave the solution to cool,
- Filter the solution and take the crystals, which will be the residue,
- Wash the crystals with distilled water then dry them between two filter papers.
Note: Do not dry the crystals in oven because it will evaporate the water of crystallization.
By Simple Distillation (For Soluble Solid/Liquid Solutions):
- Set the apparatus as shown in the diagram below,
- Turn on the Bunsen burner,
- The solvent will evaporate and rise as vapor into the condenser,
- The cold water surrounding the tube where the water is in the condenser will make the vapor condense into liquid,
- The solvent is collected in the tube or beaker on the other side of the condenser, it’s called the distillate,
- The solute is collected in the flask as powder,
- The thermometer must be where the vapor passes the measure the boiling point of the solvent.
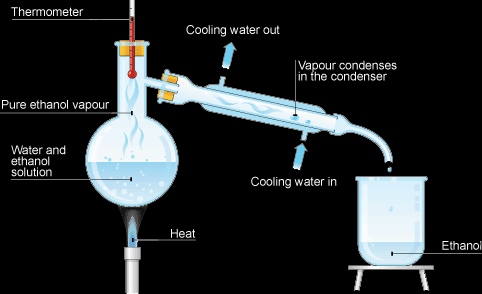
This method is ideal for distilling sea water.
Filtration (For Insoluble Solid/Liquid Mixtures):
- Set the apparatus as shown below,
- Pour the mixture into the filter funnel,
- The solvent will go through and be collected in the beaker as the filtrate,
- The insoluble solid will be collected from the funnel as the residue.
Decantation (For Insoluble Solid/Liquid Mixtures):
This method is very simple. It involves letting the insoluble solid rest at the bottom of the beaker. Then pouring the liquid in another beaker leaving the solid behind.

Centrifugation (For Insoluble Solid/Liquid Mixtures):
- Put the mixture in a test tube,
- Place the test tube in the centrifugation machine,
- Start the machine.
The centrifugation force will make the mixture separate into two layers, the liquid at the top, and solid at the bottom. They are then separated by decantation.
Separating Liquid/Liquid Mixtures:
Separating Funnel (For Immiscible Liquids):Immiscible liquids do not mix together; like oil and water. If they are put in one container, the denser liquid will settle at the bottom and the lighter one will go above it. To separate and oil and water mixture, we pour the mixture into the separating funnel. The water is denser than oil, it settles below it. The tap is opened to let the water flow into the beaker. The tap is closed when all the water is poured, the beaker is replaced by and empty one and the oil is now poured. |
 |
Fractional Distillation (For Miscible Liquids):
Fractional distillation is a method of separating a mixture of two or more liquids provided that they have different boiling points:
- The apparatus is set as in the diagram below,
- When the heat is turned on the vapor of all the liquids rises,
- The liquid with the lowest boiling point goes all the way through the glass beads and into the condenser and out on the other side as liquid. The temperature is constant during this,
- The liquids with the higher boiling points condense on the glass beads. When all of the liquid with the lowest boiling point have evaporated and collected, the temperature starts rising again. The liquid with the second lowest boiling point evaporates now, and gets collected on the other side.
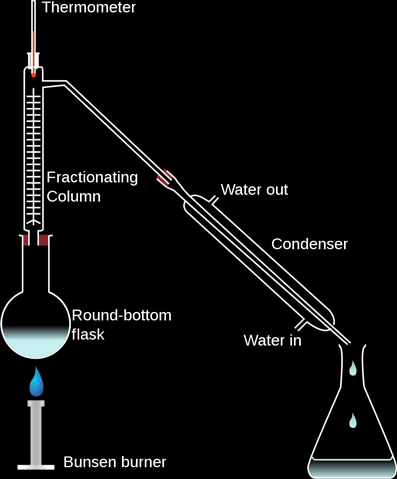
The glass beads are to provide a cool large surface area for condensation.
Fractional Distillation of Crude Oil:
Crude oil is a mixture of hydrocarbons. It is the major source of fuel. It is refined and separated into several very useful fractions by fractional distillation in a fractionating tower. The higher the fraction is obtained in the fractionating tower the lower its boiling point.
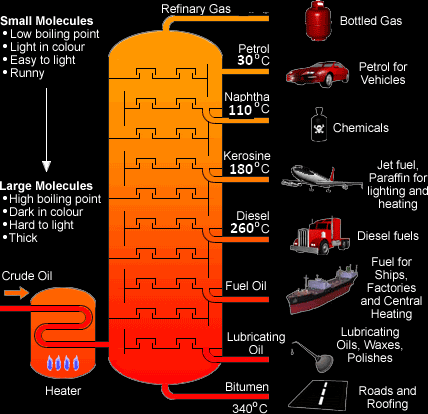
Fuel is a substance that releases energy (E.g.: Coal, Natural gas, Ethanol)
Lubricant is a substance that reduces friction between two surfaces.
Hydrocarbons are organic compounds containing carbon and hydrogen only.
Different hydrocarbons are collected at different levels according to their boiling points. The higher they are collected the lower their boiling point.
Chromatography:
Chromatography is a process used to separate and identify two or more substances from a mixture. This method depends on the solubility of the tested substances. Chromatography, for instance, is also used to find out the number of components in a drink.
Let’s say we want to find the number of colored dyes present in black ink. First we get a piece of filter paper or chromatography paper. We draw a line, in pencil, at the bottom of the paper. This line is called the base line, and the reason it is drawn in pencil is because pencil is insoluble so it won’t interfere with the solubility of the ink. Then we place a spot of the black ink on the base line. The chromatography paper is now put with its bottom soaked in a suitable solvent, which is in our case water. The chromatography paper is going to absorb the solvent, which moves upwards. When the solvent reaches the base line, the spot of black ink will dissolve in it. The solvent will keep moving upwards taking with it the black ink. The more soluble the contents of the ink the higher it will move until it can’t anymore.
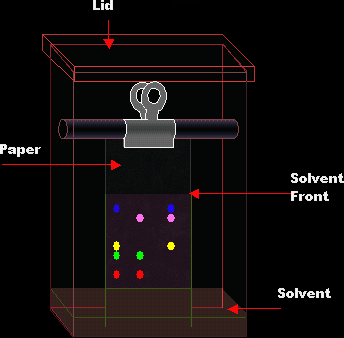
Sometimes the substance we are testing is in solid form. In this case we have to crush and dissolve it in water and filter it. We then take the filtrate and evaporate some of it water to get the most concentrated sample. Then we are ready to do the experiment.
When dealing with ethanol in concentrating the sample. We have to heat it in a water bath because it is flammable. And when we use it a solvent in chromatography, it has to be performed in a covered beaker because ethanol is volatile.
The solvent front is the furthest distance travelled by the solvent.
Sometimes, the sample is separated into colorless spots. In this case the chromatography paper is sprayed with a locating agent to that locates the spots. The number of spots indicates the number of components in the sample.
To identify the substances which were formed when the sample was separated, we measure what’s called the Rf Value. The Rf Value is the rate of the distance travelled by the solute (the spot) to the distance travelled by the solvent line. It’s calculated by measuring the distance travelled by the spot (Distance1) from the base line, measuring the distance from the base line to the solvent front (Distance2), and dividing Distance1 by Distance2.
![]()
This value is always less than one because the distance travelled by the solvent is always larger than the distance travelled by the spot. Each substance has a different Rf Value.
Chromatography can be used to test purity of substances. If a substance gives only one spot, it means it is pure because it contains one substance.
If two spots have the same Rf value they are made of the same substance.