Electricity in Chemistry
Electrolysis is a process of breaking down a compound by electricity. An electric current is the flow of charged particles.
Conductivity:
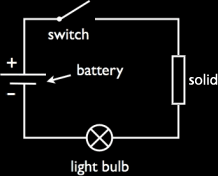
In solids, substances that conduct electricity are called Conductors. These are mostly metals and graphite. This is because metals and graphite contain free electrons in their structures to carry the charge. The solids which do not conduct electricity are called Insulators. To test a solid for electrical conductivity, we put it an electrical circuit like the one below. If the bulb lights or the ammeter gives a reading, then the solid is a conductor. For liquids however, the ones that conduct electricity are called electrolytes. The ones that do not are called non-electrolytes. Electrolytes include acids, alkalis, and ionic compounds in molten or aqueous form. |
 |
The Electrolysis Cell:
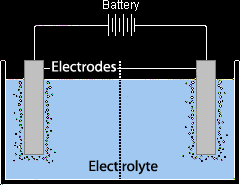
The electrolysis cell is a battery each pole connected to an electrode and both electrodes are dipped in the liquid to be electrolysed.
There are two types of electrodes, active electrodes and inert electrodes. Active electrodes take place in the process its self. Inert electrodes are just there to conduct the current without interfering. Inert electrodes can be either graphite or platinum but graphite is more widely used because it’s cheaper. Inert electrodes are always used in electrolysis; active ones are used in electroplating. |
 |
How It Works:
Electrolysis separates an ionic compound back to the elements that form it. For example by electrolysis we can obtain sodium and chlorine from sodium chloride.
When the current is turned on, the negative ion in the electrolyte gets attracted to the positive electrode because they are oppositely charged. When this happens, the negative ion loses the electrons it gained from the positive ion during bond formation and becomes an atom. The electrons lost are transferred through the wire in the outer circuit from the anode to the cathode. At the same time, the positive ion from the electrolyte is attracted to the cathode, where it gains the electrons lost by the negative ion and becomes an atom too.
In ionic compounds the positive ion is a metal and it is collected at the cathode. And the negative ion is a non-metal and collected at the anode.
The electrons are transferred from the anode to the cathode through the wires.
The electrolyte is an ionic compound either in its molten or aqueous form. Ionic compounds conduct electricity only when they are in these forms because they contain free mobile ions which can carry the current but they don’t in solid form.
Electrolysis of Molten Ionic Compounds:
An idealized cell for the electrolysis of sodium chloride is shown in the figure below. A source of direct current is connected to a pair of inert electrodes immersed in molten sodium chloride. Because the salt has been heated until it melts, the Na+ ions flow toward the negative electrode and the Cl- ions flow toward the positive electrode.
Negative electrode (cathode): Na+ + e- → Na Cl- ions that collide with the positive electrode are oxidized to Cl2 gas, which bubbles off at this electrode. Positive electrode (anode): 2Cl- → Cl2 + 2e- The net effect of passing an electric current through the molten salt in this cell is to decompose sodium chloride into its elements, sodium metal and chlorine gas. |
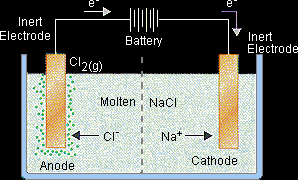 |
2NaCl(l) → 2 Na(l) + Cl2(g)
This example explains why the process is called electrolysis. The suffix -lysis comes from the Greek stem meaning to loosen or split up. Electrolysis literally uses an electric current to split a compound into its elements.
Electrolysis of Aqueous Ionic Compounds:
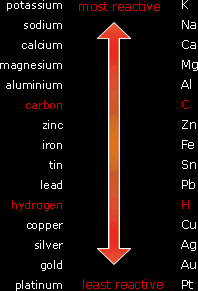
Electrolysing an ionic compound in its solution is very much different to electrolysing it when it’s molten. This is because in a solution we have 4 ions, H+ and OH- from water and a positive and a negative ion from the compound. But only one type of ions gets discharged at each electrode. For the positive ions, the one that gets discharged at the cathode is the least reactive one. This is because least reactive elements have more tendencies to be an atom. So if the ion from the ionic compound is above hydrogen in the reactivity series (more reactive), H+ gets discharged at the anode And if the ion from the compound is below hydrogen in the reactivity series (less reactive), this ion gets discharged at the cathode. So for example if we are electrolysing aqueous sodium chloride, H+ ions will get discharged at the cathode because sodium is more reactive than hydrogen. And if we are electrolysing aqueous copper iodide, Cu2+ ions will get discharged at the cathode because copper is less reactive than hydrogen. |
 |
For the negative ions however it is different. Oxygen from OH- from water is always discharged at the anode except in one case, this is if the other negative ion is a halide. If oxygen from OH- is discharged, the equation will be:
4OH- - 4e → O2 + H2O
If the other negative ion is a halide, there are two probabilities:
- Oxygen from OH- gets discharged at the cathode,
- The halide ion gets discharged at the cathode.
It all depends on the concentration of the halide. If the electrolyte is a concentrated solution, then there are many of the halide ions, more than OH-. So the halide ion gets discharged at the cathode. If the electrolyte is a dilute solution, then there are more OH- ions than halide ions, so oxygen from OH- gets discharged.
So for example if the electrolyte is a concentrated solution of sodium chloride, hydrogen gas is formed at the cathode because hydrogen is less reactive than sodium. And chlorine gas is formed at the anode because the solution is concentrated.
If the electrolyte is a dilute solution of silver sulfate, silver is formed at the cathode because it is less reactive than hydrogen and oxygen gas is formed at the anode.
Electrolysis of Brine (concentrated aqueous sodium chloride):
The ions present in the electrolyte are H+ and OH- from water and Na+ and Cl- from sodium chloride.
Since sodium is more reactive than hydrogen, the H+ ions will be discharged at the cathode and hydrogen gas will evolve. And because the solution is concentrated, Cl- will be discharged and chlorine gas will evolve. But keep in mind that chlorine is soluble in water, it would take time for it to evolve and some oxygen can be formed too. Gases should be collected in an inverted measuring cylinder.
This leaves us with two other ions, Na+ and OH-. They bond together forming sodium hydroxide which is an alkali and extracted later.
At the cathode: 2H+ + 2e- → H2
Hydrogen gas evolves. Observation is bubbles of colorless gas.
Test to make sure by approaching a lighted splint, if positive it will burn with a pop sound.
At the anode: 2Cl- - 2e- → Cl2
Chlorine gas evolves. Observation is bubbles of green gas. Test to make sure by approaching a damp blue litmus paper, if positive it will turn red then bleach.
2NaCl + 2H2O → H2 + Cl2 + 2NaOH
Electrolysing Brine in a Membrane Cell:
In order to obtain the purest products possible, a membrane cell like the one below is used.
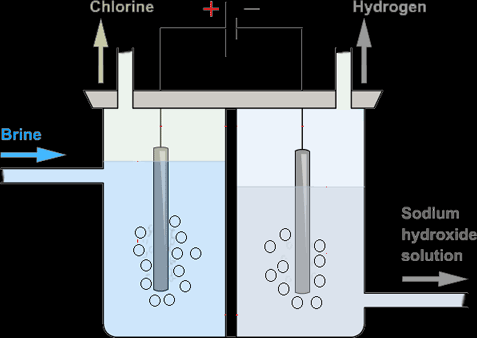
In this cell, there is a membrane between both electrodes that separate Cl- ions from OH- ions. When the solution is added to the membrane, the membrane allows Na+, H+ and OH- ions to pass to the cathode chamber and Cl- stays in the anode chamber. Cl- gets discharge while the OH- is trapped in the cathode chamber and can’t pollute the chlorine gas being collected. H+ gets discharged at the anode and collected. Na+ and OH- bond together forming sodium hydroxide which is extracted from the bottom of the cathode chamber.
Electrolysis of Copper(II) Sulfate Solution:
Ions present in the electrolyte are H+ and OH- from water and Cu+2 and SO4- from copper(ii) sulfate.
At the Cathode: 4OH- - 4e- → O2 + 2H2O
Oxygen from OH- ions is formed. Bubbles of colorless gas are formed. To test for oxygen, approach a glowing splint, if positive it relights.
At the anode: Cu+2 + 2e- → Cu
Cu+2 are discharged because copper is less reactive than hydrogen. A red brown metal is formed.
This leaves us with H+ and SO4- ions which bond together forming sulfuric acid.
Note: a copper sulfate solution is blue in color. In this process, the blue color gradually fades away because copper sulfate is being broken down. The solution becomes acidic becomes acidic because sulfuric acid is formed.
-
Note:
- When a product of electrolysis is a halogen, bear in mind that it is soluble in water so it could take time to evolve and might be replaced by oxygen from OH-.
- When a product of electrolysis is a halogen, perform it in a fume cupboard because halogens are toxic.
Electrolysis and Refining:
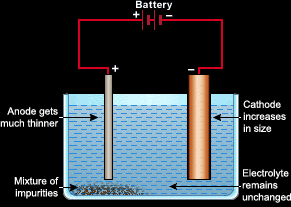
Electrolysis can be used to refine metals. For example if we have a sample of impure copper that we want to refine, we set up a unique electrolysis cell to do that.
- The cathode will be made of pure copper.
- The anode will be made of the impure copper sample.
- The electrolyte will be a solution of a copper salt (copper sulfate/nitrate)
When the battery is switched on, the sea of delocalized electrons in impure copper sample will be absorbed by the battery. The impure copper then will turn into copper ions and fall into the electrolyte. Now the electrolyte has copper ions from the copper salt and the anode. When the electrons reach the cathode, the copper ions which fell from the anode will get attracted to the cathode and take their electrons back turning into atoms.
The anode gradually gets thinner and disappears because the copper ions are falling of it. So for generally, for refining a metal, the electrolysis cell must be set up as follows:
|
 |
Electroplating:
Electroplating is covering a metal object with another metal by electrolysis.
Purposes of electroplating are:
- To give the object a protective layer from corrosion
- To give the object a shiny better look
The idea of the electroplating cell is very similar to that of refining:
- The Anode is the metal to electroplate with
- The cathode is the object to be electroplated
- The electrolyte is a salt solution of the metal to electroplate with
When the current is turned on, the atoms of the metal of the anode will become ions and fall into the solution. The electrons are transferred from the anode to the cathode in the outer circuit. When electrons reach the cathode, the metal ions in the electrolyte get attracted to the surface of the object covering it completely, thus it gets electroplated.
-
Note:
- The object to be electroplated must be rubbed and cleaned with sand paper to remove any stains that won’t let the metal cover the whole object firmly
- The object to be electroplated must be dipped completely in the electrolyte and rotated continuously to make sure all the object gets covered uniformly.
- The object to be electroplated must be made of an electrical conductor.
Example:
If we want to electroplate a steel fork with silver:
- The anode will be a pure sample of silver,
- The cathode will be the fork,
- The electrolyte will be a silver nitrate solution.
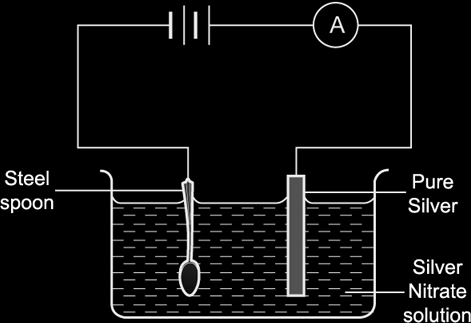
Anode:
Equation: Ag – e- → Ag+
Observation: Gets thinner.
Cathode:
Equation: Ag+ + e- → Ag
Observation: Gets covered with silver layer, increases in size.
Simple Chemical Cell:
A simple cell is a system that converts chemical energy into electrical energy. A simple cell is made of two metals (electrodes), one more reactive than the other, connected together by a wire and dipped in an electrolyte. If a light bulb is inserted between the two electrodes, it will light up.
If cell consists of a zinc electrode and a copper electrode dipped in sulfuric acid, this is how it works:
|
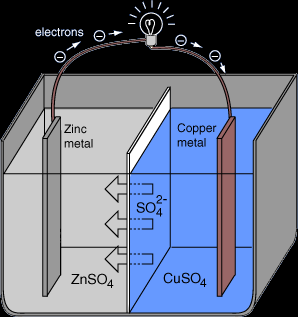 |
At the negative pole (Zinc Anode):
Equation: Zn - 2e- → Zn+2
Observation: Anode gets thinner
At the positive pole (Copper Cathode):
Equation: 2H+ + 2e- → H2
Observation: Bubbles of colorless gas.
The bulb stops glowing when H+ ions are finished or when the anode is used up.
The larger the difference in reactivity between the two metals the larger the voltage produced.