Carbonates
Carbonates are salts of carbonic acids (H2CO3). Carbonates are very useful salts, specially calcium carbonate (CaCO3). Sources of Calcium Carbonate:Calcium carbonate can be found in large amounts in the Peak District. It is found as a type of rocks called limestone near rivers. Forms of Calcium Carbonate:Limestone is not the only form of calcium carbonate. Marble and chalk are also other forms of this valuable salt. Chalk is made of shells of marine algae. Marble on the other hand, is a metaphoric rock made of limestone at high pressure. |
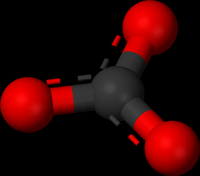 |
| (Ball-and-stick model of the carbonate ion, CO32−) |
Uses of Calcium Carbonate:
Calcium carbonate has numerous uses. You have previously studied one of them which is helping in the extraction of iron from its ore. Another one of these is the manufacture of cement. In this process, limestone or chalk is mixed with clay and heated in a rotary kiln. The substance in the mixture react producing cement which is a mixture of calcium aluminate (Ca(AlO2)2) and calcium silicate (CaSiO3). This is then made into powder. When it is used, it is sprayed with water make its particles hold tight.
Manufacture of Lime:
One of the industrial uses of calcium carbonate is the manufacturing of lime from it. Lime is calcium oxide salt. This process takes place in a device called lime kiln and it is based on the thermal decomposition of calcium carbonate. Limestone is inserted in the kiln and heating starts. At the bottom of the kiln air is being blown in. this is also where lime is collected. The other product of this reaction, carbon dioxide gas, evolves and escapes at the top of the kiln.
| CaCO3 | ⇌ | Cao | + | CO2 |
| (Limestone) | (Lime) | (Carbon Dioxide) |
Uses of Lime:
Lime can be used to neutralise soil acidity in farms. This is because it is a basic oxide. Slaked lime (Calcium hydroxide; Ca(OH)2) is also a basic oxide can be used as an alternative to lime for neutralising soil acidity. Another use of lime is neutralising sulphur dioxide waste in power stations. This is because sulphur dioxide is an acidic oxide while lime is a basic one. This process is called desulphurisation which you have studied earlier.