Bonding Structures
In the previous chapter, we studied that noble gases are stable and unreactive because they have a full energy shells. All other elements are seeking this property too. They all want to have full outer most energy shells; this is why reactions take place. For example if an atom has 6 electrons in its outer most shell, it tries to gain two electrons from other atoms to complete the shell with 8 electrons. We have two types of bonds, ionic and covalent bonds.
Ionic Bonding:
This type of bonding is based on the electrostatic force of attraction between the ions in the molecule. For example, when sodium, which is in group one and has one electron in its outer most shell, reacts with chlorine, which is in group 7 and has 7 electrons in its outer most shell, the sodium gets rid of the only electron in its outer shell, thus the sodium atom will have its second most outer shell which is full become its most outer shell forming a positive ion. The electron which is lost by the sodium atom is gained by the chlorine atom to 8 electrons, thus filling its outer most shell and becoming a negative ion. This electron transfer causes the electrostatic force of attraction which holds the oppositely charged ions together in a molecule. When an atom becomes an ion, it gets the properties of the noble gas which is nearest to it in the periodic table. Ionic bonds are only formed between metals and non-metals.
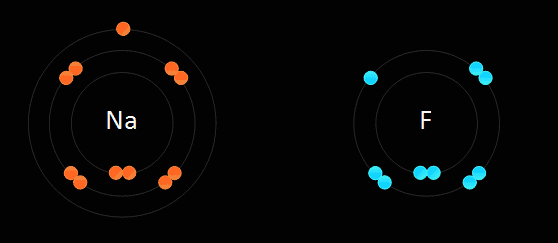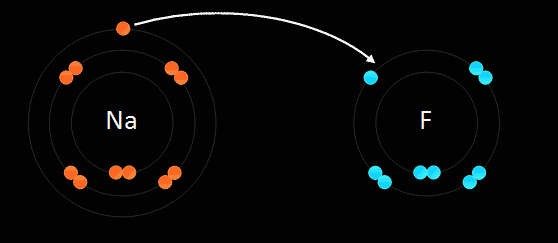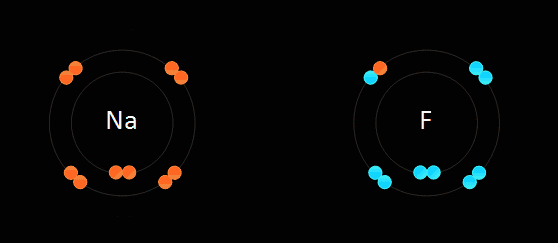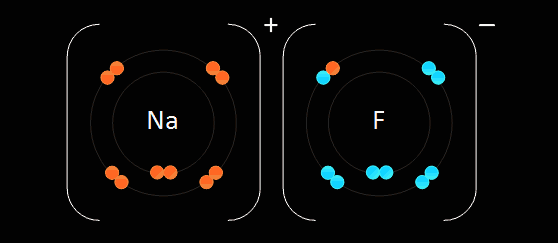
Formulae of Ionic Compounds:
To find the formula for an ionic compound, we use a method similar to cross multiplication. We multiply the valency (valency is the number of electrons an atom loses or gains to form an ion) to the other atom as shown below:
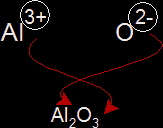
Properties of Ionic Compounds:
- Hard solids at room temperature,
- High melting and boiling points because of strong attraction forces,
- When solid they are electrical insulators but conduct electricity when molten or aqueous,
- Water soluble.
Covalent Bonding:
This type of bonding occurs between non-metals only. In order to obtain a full outer most energy shell, the atoms tend to share the electrons of their outer most energy shell, some or all of them.
Example #1: A hydrogen molecule:
Each hydrogen atoms has 1 electron in its outer most shell. When it bonds covalently, it shares this electron with another hydrogen atom, which also shares its only valence electron. This causes each atom in the molecule to have 2 electrons in its outer most shell, which is also the 1st and only shell; this means it is holding the maximum number of electrons making it stable.
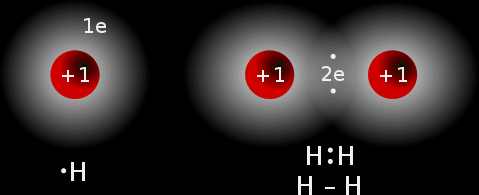
If two atoms each share one electron, it is called a single covalent bond, if each shares two electrons; it is a double covalent bond. The single covalent bond above can be represented by H - H.
A double covalent bond between two carbon atoms can be represented b C = C.
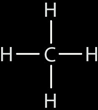
Example #2: Methane molecule:Each carbon atom has 4 valence electrons, this means it can bond with 4 hydrogen atoms (1 valence electron) covalently as shown on the right. This molecule can also be represented like this: |
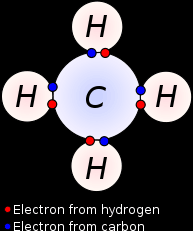 |
Types of Covalent Structures:
There are two types of covalent structures:
- Simple Molecular Structure
- Giant Molecular Structure
Simple Molecular Structure:
They are simple and contain only a few atoms in one molecule. Covalent bonds between the atoms within a molecule (intermolecular bonds) are strong but they have weak bonds between molecules (intermolecular bonds). These forces increase as the size of the molecule increases.
Giant Molecular Structure:
They are also known as macromolecular structures. One molecule contains hundreds of thousands of atoms. They have extremely strong bonds between the atoms (intermolecular bonds).
Properties of Covalent Compounds:
- Simple molecular structures are usually gases or liquids and sometimes solids with low melting points; this is because of weak forces of attraction between the molecules which can be broken easily.
- Giant molecular structures have very high melting points because the whole structure is held together with very strong covalent bonds.
- Most of them do not conduct electricity
- Most of them are insoluble in water
Allotropes of Carbon:
What are allotropes?
When an element exists in several physical forms of the same state, it is said to exhibit allotropy. Each form of this element is an allotrope. Lots of elements exhibit allotropy. Carbon has two very popular allotropes, diamond and graphite. Diamond and graphite are both made of carbon only. However, they look very different and have different physical properties. They are both giant molecular structures.
Diamond:
In diamond’s structure, each carbon atom is covalently bonded to four other carbon atoms by very strong bonds forming a 3D tetrahedral shape. The physical properties of diamond:
|
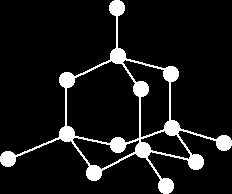 |
|
|
Graphite:
In the graphite structure, each carbon atom is strongly bonded covalently to three other carbon atoms forming layers of linked hexagons. Each layer acts as a molecule, the intermolecular forces between the layers is very weak allowing layers to slide over each other. This makes graphite a good lubricant. The physical properties of graphite:
|
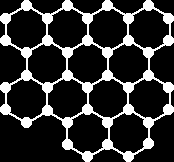 |
|
|
Metallic Structures:
Pure metals have a very unique structure. Each atom lets go of the valence electrons and become a positive ion. These electrons altogether form a sea of delocalized electrons. Since the electrons are negatively charged and the ions are positively charged, an electrostatic force of attraction is formed between the sea of delocalized electrons and layers of positive ions. The metallic lattice is the regular arrangement of positive ions embedded in a sea of delocalized electrons. The metallic bond is the electrostatic force of attraction between the layers of positive ions and the sea of delocalized electrons. |
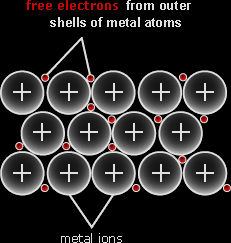 |
Properties of metals:
|
|
Alloys:
An alloy is a mixture of metals or metals and non-metals. Sometimes, and alloy is better than a metal because they have better properties. They are harder, more resistant to corrosion and have a more attractive appearance than the metals they are formed of.
Alloys are harder than metals because they have different sized atoms which prevent the layers from sliding over each other.
And alloy is made by heating the metals or metals and non-metals together until they all melt, and leaving them to cool mixed.
Examples of alloys and their content:
- Brass: Copper-Zinc,
- Bronze: Copper-Tin,
- Steel: Iron-Carbon,
- Stainless Steel: Iron-Carbon-Chromium-Nickel.