Acids, Bases and Salts
All substances are acidic, neutral or basic (alkaline). How acidic or basic a substance is shown by its pH. There are several other ways by which we could find out whether a substance is acidic, neutral or basic.
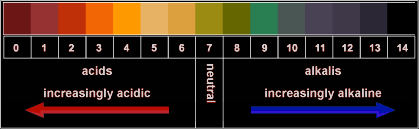
pH Scale:
This is a scale that runs from 0 to 14. Substances with a pH below 7 are acidic. Substances with pH above 7 are basic. And those with pH 7 are neutral.
Indicators:
Indicators are substances that identify acidity or alkalinity of substances. They cannot be used in solid form.
Universal Indicator:
This is a substance that changes color when added to another substance depending on its pH. The indicator and the substance should be in aqueous form.
Litmus Paper or Solution:
This indicator is present in two colors: red and blue. We use blue litmus if we want test a substance for acidity. We use red litmus if we want to test a substance for alkalinity. Its results are:
- Acids: Turns blue litmus paper/ solution red,
- Bases: Turns red litmus paper/ solution blue,
- Neutral: if it is used as paper the color doesn’t change. If it is used as solution it turns purple.
Note: use damp litmus paper if testing gases.
Phenolphthalein:
This is an indicator that is used to test for alkalinity because it is colorless if used with an acidic or neutral substance and it is pink if it is used with a basic substance.
Methyl Orange:
This indicator gives fire colors: Red with acids, yellow with neutrals and orange with bases.
Acids:
Acids are substances made of a hydrogen ion and non-metal ions. They have the following properties:
- They dissolve in water producing a hydrogen ion H+,
- They have a sour taste,
- Strong ones are corrosive,
- Their pH is less than 7.
All acids must be in aqueous form to be called an acid. For example Hydrochloric acid is hydrogen chloride gas dissolved in water. The most common acids are:
- Hydrochloric acid HCl,
- Sulphuric Acid H2SO4,
- Nitric Acid HNO3,
- Cirtric Acid,
- Carbonic Acid H2CO3.
Strength of Acids:
One of the most important properties of acids is that it gives hydrogen ion H+ when dissolved in water. This is why the amount of H+ ions the acid can give when dissolved in water is what determines its strength. This is called ionization or dissociation. The more ionized the acid is the stronger it is, the lower its pH. The more H+ ions given when the acid is dissolved in water the more ionized the acid is.
Strong Acids:
|
Weak Acids:
|
Hydrochloric acid is a strong acid. When it is dissolved in water all HCl molecules are ionized into H+ and Cl- ions. It is fully ionized.
Ethanoic acid has the formula CH3COOH. It is a weak acid. When it is dissolved in water, only some of the CH3COOH molecules are ionized into CH3COO- and H+ ions. It is partially ionized.
Note: Acids with pH 3 or 4 can be considered moderate in strength.
Solutions of strong acids are better conductors of electricity than solutions of weak acids. This is because they contain much more free mobile ions to carry the charge.
Concentrated acids are not necessarily strong. The concentration of an acid only means the amount of molecules of the acid dissolved in water. Concentrated acids have a large amount of acid molecules dissolved in water. Dilute acids have a small amount of acid molecules dissolved in water. Concentration is not related to strength of the acids. Strong acids are still strong even if they are diluted. And weak acids are still weak even if they are concentrated.
Bases:
Bases are substances made of hydroxide OH- ions and a metal. Bases can be made of:
- Metal hydroxide (metal ion & OH- ion)
- Metal oxides
- Metal carbonates (metal ion & CO32-)
- Metal hydrogen carbonate (Bicarbonate)
- Ammonium hydroxide (NH4OH)
- Ammonium Carbonate ((NH4)2CO3)
Properties of bases:
- Bitter taste
- Soapy feel
- Have pH’s above 7
- Strong ones are corrosive
Some bases are water soluble and some bases are water insoluble. Water soluble bases are also called alkalis.
Like acids, alkalis' strength is determined by its ability to be ionized into metal and hydroxide OH- ions. Completely ionized alkalis are the strongest and partially ionized alkalis are the weakest. Ammonium hydroxide is one of the strongest alkalis while weak alkalis include the hydroxides of sodium, potassium and magnesium.
Types of Oxides:
Basic Oxides |
Amphoteric Oxides |
Acidic Oxides H2O (neutral oxides) |
Salts:
A salt is a neutral ionic compound. Salts are one of the products of a reaction between an acid and a base. Salts are formed in reactions I n which the H+ ion from the acid is replaced by any other metal ion. Some salts are soluble in water and some are insoluble.
Soluble Salts: |
Insoluble Salts: |
Preparing Soluble Salts:
Displacement Method (Excess Metal Method):
Metal + Acid → Salt + Hydrogen
Note: this type of method is suitable to for making salts of moderately reactive metals because highly reactive metals like K, Na and Ca will cause an explosion. This method is used with the MAZIT (Magnesium, Aluminum, Zinc, Iron and Tin) metals only.
Example: set up an experiment to obtain magnesium chloride salt.
Mg + 2HCl → MgCl2 + H2
- Add 100 cm3 of dilute hydrochloric acid to a beaker
- Add excess mass of powdered magnesium
- When the reaction is done, filter the mixture to get rid of excess magnesium (residue)
- The filtrate is magnesium chloride solution
- To obtain magnesium chloride powder, evaporate the solution till dryness
- To obtain magnesium chloride crystals, heat the solution while continuously dipping a glass rod in the solution
- When you observe crystals starting to form on the glass rod, turn heat off and leave the mixture to cool down slowly
- When the crystals are obtained, dry them between two filter papers
Observations of this type of reactions:
- Bubbles of colorless gas evolve (hydrogen). To test approach a lighted splint if hydrogen is present it makes a pop sound
- The temperature rises (exothermic reaction)
- The metal disappears
You know the reaction is over when:
- No more gas evolves
- No more magnesium can dissolve
- The temperature stops rising
- The solution becomes neutral
Proton Donor and Acceptor Theory:
When an acid and a base react, water is formed. The acid gives away an H+ ion and the base accepts it to form water by bonding it with the OH- ion. A hydrogen ion is also called a proton this is why an acid can be called Proton Donor and a base can be called Proton Acceptor.
Neutralization Method:
Acis + Base → Salt + Water
Note: This method is used to make salts of metals below hydrogen in the reactivity series. If the base is a metal oxide or metal hydroxide, the products will be salt and water only. If the base is a metal carbonate, the products will be salt, water and carbon dioxide.
Type 1:
Acid + Metal Oxide → Salt + Water
To obtain copper sulfate salt given copper oxide and sulfuric acid:
CuO + H2SO4 → CuSO4 + H2O
- Add 100 cm3 of sulfuric acid to a beaker
- Add excess mass of Copper oxide
- When the reaction is over, filter the excess copper oxide off
- The filtrate is a copper sulfate solution, to obtain copper sulfate powder evaporate the solution till dryness
- To obtain copper sulfate crystals, heat the solution white continuously dipping a glass rod in it
- When you observe crystals starting to form on the glass rod, turn heat of and leave the mixture to cool down slowly
- When you obtain the crystals dry them between two filter papers
Observations of this reaction:
- The amount of copper oxide decreases
- The solution changes color from colorless to blue
- The temperature rises
- You know the reaction is over when
- No more copper oxide dissolves
- The temperature stops rising
- The solution become neutral
Type 2:
Acid + Metal Hydroxide → Salt + Water
to obtain sodium chloride crystals given sodium hydroxide and hydrochloric acid:
HCl + NaOH → NaCl + H2O
- Add 100 cm3of dilute hydrochloric acid to a beaker
- Add excess mass of sodium hydroxide
- When the reaction is over, filter the excess sodium hydroxide off
- The filtrate is sodium chloride solution, to obtain sodium chloride powder, evaporate the solution till dryness
- To obtain sodium chloride crystals, hear the solution while continuously dipping a glass rod in it
- When crystals start to form on the glass rod, turn heat off and leave the mixture to cool down slowly
- When the crystals are obtained, dry them between two filter papers
Observations:
- Sodium hydroxide starts disappearing
- Temperature rises
You know the reaction is over when:
- The temperature stops rising
- No more sodium hydroxide can dissolve
- The pH of the solution becomes neutral
Type 3:
Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
To obtain copper sulfate salt given copper carbonate and sulfuric acid:
CuCO3 + H2SO4 → CuSO4 + H2O + CO2
- Add 100 cm3 of dilute sulfuric acid to a beaker
- Add excess mass of copper carbonate
- When the reaction is over, filter excess copper carbonate off
- The filtrate is a copper sulfate solution, to obtain copper sulfate powder evaporate the solution till dryness
- To obtain copper sulfate crystals, heat the solution white continuously dipping a glass rod in it
- When you observe crystals starting to form on the glass rod, turn heat of and leave the mixture to cool down slowly
- When you obtain the crystals dry them between two filter papers
Observations:
- Bubbles of colorless gas (carbon dioxide) evolve, test by approaching lighted splint, if the CO2 is present the flame will be put off
- Green Copper carbonate starts to disappear
- The temperature rises
- The solution turns blue
You know the reaction is finished when:
- No more bubbles are evolving
- The temperature stops rising
- No more copper carbonate can dissolve
- The pH of the solution becomes neutral
Titration Method:This is a method to make a neutralization reaction between a base and an acid producing a salt without any excess. In this method, the experiment is preformed twice, the first time is to find the amounts of reactants to use, and the second experiment is the actual one. 1st Experiment:
|
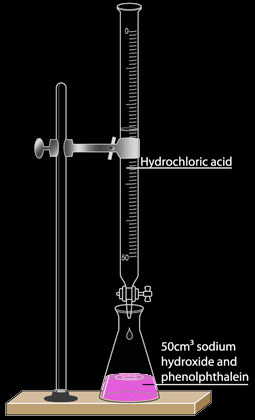 |
Preparing Insoluble Salts:
Precipitation Method:
A precipitation reaction is a reaction between two soluble salts. The products of a precipitation reaction are two other salts, one of them is soluble and one is insoluble (precipitate).
Example: To obtain barium sulfate salt given barium chloride and sodium sulfate:
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Ionic Equation: Ba2+ + SO42- → BaSO4
- Add the two salt solutions in a beaker
- When the reaction is over, filter and take the residue
- Wash the residue with distilled water and dry it in the oven
Observations:
- Temperature increases
- An insoluble solid precipitate (Barium sulfate) forms
You know the reaction is over when:
- The temperature stops rising
- No more precipitate is being formed
Controlling Soil pH:
If the pH of the soil goes below or above 7, it has to be neutralized using an acid or a base. If the pH of the soil goes below 7, calcium carbonate (lime stone) is used to neutralize it. The pH of the soil can be measured by taking a sample from the soil, crushing it, dissolving in water then measuring the pH of the solution.
Colors of Salts:
| Salt | Formula | Solid | In Solution |
|---|---|---|---|
| Hydrated copper sulfate | CuSO4.5H2O | Blue crystals | Blue |
| Anhydrous copper sulfate | CuSO4 | White powder | Blue |
| Copper nitrate | Cu(NO3)2 | Blue crystals | Blue |
| Copper chloride | CuCl2 | Green | Green |
| Copper carbonate | CuCO3 | Green | Insoluble |
| Copper oxide | CuO | Black | Insoluble |
| Iron(II) salts | E.g.: FeSO4, Fe(NO3)2 | Pale green crystals | Pale green |
| Iron(III) salts | E.g.: Fe(NO3)3 | Reddish brown | Reddish brown |
Tests for Gases:
| Gas | Formula | Tests |
|---|---|---|
| Ammonia | NH3 | Turns damp red litmus paper blue |
| Carbon dioxide | CO2 | Turns limewater milky |
| Oxygen | O2 | Relights a glowing splint |
| Hydrogen | H2 | ‘Pops’ with a lighted splint |
| Chlorine | Cl2 | Bleaches damp litmus paper |
| Nitrogen dioxide | NO2 | Turns damp blue litmus paper red |
| Sulfur dioxide | SO2 | Turns acidified aqueous potassium dichromate(VI) from orange to green |
Tests for Anions:
| Anion | Test | Result |
|---|---|---|
| Carbonate (CO32-) | Add dilute acid | Effervescence, carbon dioxide produced |
Chloride (Cl-) |
Acidify with dilute nitric acid, then add aqueous silver nitrate |
White ppt. |
| Iodide (I-) (in solution) |
Acidify with dilute nitric acid, then add aqueous silver nitrate |
Yellow ppt. |
| Nitrate (NO3-) (in solution) |
Add aqueous sodium hydroxide, then aluminium foil; warm carefully |
Ammonia produced |
| Sulfate (SO42-) | Acidify, then add aqueous barium nitrate | White ppt. |
Tests for aqueous cations:
| Cation | Effect of aqueous sodium hydroxide | Effect of aqueous ammonia |
|---|---|---|
| Aluminium (Al3+) | White ppt., soluble in excess giving a colourless solution |
White ppt., insoluble in excess |
| Ammonium (NH4+) | Ammonia produced on warming | – |
| Calcium (Ca2+) | White ppt., insoluble in excess | No ppt. or very slight white ppt. |
| Copper (Cu2+) | Light blue ppt., insoluble in excess | Light blue ppt., soluble in excess, giving a dark blue solution |
| Iron(II) (Fe2+) | Green ppt., insoluble in excess | Green ppt., insoluble in excess |
| Iron(III) (Fe3+) | Red-brown ppt., insoluble in excess | Red-brown ppt., insoluble in excess |
| Zinc (Zn2+) | White ppt., soluble in excess, giving a colourless solution |
White ppt., soluble in excess, giving a colourless solution |