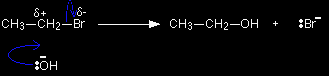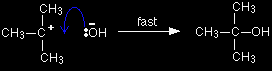THE REACTIONS OF ARYL HALIDES (HALOGENOARENES)This page only looks at one aspect of the chemistry of the aryl halides such as chlorobenzene - the fact that they are very unreactive compared with halogenoalkanes (haloalkanes or alkyl halides). This is the only bit of their chemistry asked for by any UK A level syllabuses. Nucleophilic substitution reactionsA nucleophile can be either a negative ion or a molecule which carries a partial negative charge somewhere on it. On this page, we are just going to be looking at a simple nucleophile - a hydroxide ion. A nucleophilic substitution reaction is one in which a part of a molecule is replaced after it has been attacked by a nucleophile. |
|
|
Note: To make sense of this page, you will have to understand how nucleophilic substitution happens. Follow this link to the introductory page on nucleophilic substitution reactions if you aren't absolutely confident about this. It would also be worthwhile looking at the page specifically about nucleophilic substitution by hydroxide ions. Both of these pages deal with nucleophilic substitution in the halogenoalkanes. The rest of this page is going to be a comparison with these reactions, and so it is important that you understand them. Use the BACK button (or HISTORY file or GO menu) on your browser to return to this page later. |
|
Nucleophilic substitution in the halogenoalkanes Here is a quick summary of the two ways that halogenoalkanes can react with hydroxide ions. We'll compare these with the aryl halides afterwards. The two different ways in which these reactions can happen depends on what kind of halogenoalkane you are talking about. Here is the mechanism for the reaction involving bromoethane - a primary halogenoalkane. A hydroxide ion attacks the slightly positive carbon atom and pushes off the bromine as a bromide ion.
A tertiary halogenoalkane reacts differently. The mechanism this time involves an initial ionisation of the halogenoalkane:
. . . followed by a very rapid attack by the hydroxide ion on the carbocation (carbonium ion) formed:
|
|
|
Note: If this isn't all completely obvious to you, you should have followed (or spent more time on) the links above! |
|
Nucleophilic substitution in the aryl halides Simple aryl halides like chlorobenzene are very resistant to nucleophilic substitution. It is possible to replace the chlorine by -OH, but only under very severe industrial conditions - for example at 200°C and 200 atmospheres. In the lab, these reactions don't happen. There are two reasons for this - depending on which of the above mechanisms you are talking about. The extra strength of the carbon-halogen bond in aryl halides The carbon-chlorine bond in chlorobenzene is stronger than you might expect. There is an interaction between one of the lone pairs on the chlorine atom and the delocalised ring electrons, and this strengthens the bond. |
|
|
Note: This is explained in more detail (with some diagrams) on the introduction to aryl halides page. Use the BACK button on your browser to return to this page if you follow this link. |
|
Both of the mechanisms above involve breaking the carbon-halogen bond at some stage. The more difficult it is to break, the slower the reaction will be. Repulsion by the ring electrons This will only apply if the hydroxide ion attacked the chlorobenzene by a mechanism like the first one described above. In that mechanism, the hydroxide ion attacks the slightly positive carbon that the halogen atom is attached to. If the halogen atom is attached to a benzene ring, the incoming hydroxide ion is going to be faced with the delocalised ring electrons above and below that carbon atom. The negative hydroxide ion will simply be repelled. That particular mechanism is simply a non-starter!
|
|