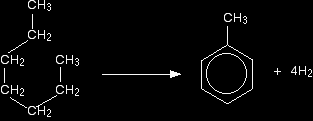MANUFACTURING ARENESThis page looks at the manufacture of arenes such as benzene and methylbenzene (toluene) by the catalytic reforming of fractions from petroleum (crude oil). Catalytic reformingWhat is reforming? Reforming takes straight chain hydrocarbons in the C6 to C8 range from the gasoline or naphtha fractions and rearranges them into compounds containing benzene rings. Hydrogen is produced as a by-product of the reactions. For example, hexane, C6H14, loses hydrogen and turns into benzene. As long as you draw the hexane bent into a circle, it is easy to see what is happening.
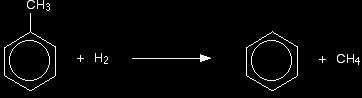
Similarly, methylbenzene (toluene) is made from heptane:
The process The feedstock The feedstock is a mixture of the naphtha or gasoline fractions and hydrogen. The hydrogen is there to help prevent the formation of carbon by decomposition of the hydrocarbons at the high temperatures used. The carbon would otherwise contaminate the catalyst. The catalyst A typical catalyst is a mixture of platinum and aluminium oxide. With a platinum catalyst, the process is sometimes described as "platforming". Temperature and pressure The temperature is about 500°C, and the pressure varies either side of 20 atmospheres. Converting some of the methylbenzene into benzene Methylbenzene is much less commercially valuable than benzene. The methyl group can be removed from the ring by a process known as "dealkylation". The methylbenzene is mixed with hydrogen at a temperature of between 550 and 650°C, and a pressure of between 30 and 50 atmospheres, with a mixture of silicon dioxide and aluminium oxide as catalyst.
|