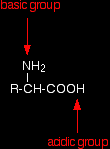
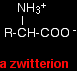
THE ACID-BASE BEHAVIOUR OF AMINO ACIDSThis page looks at what happens to amino acids as you change the pH by adding either acids or alkalis to their solutions. For simplicity, the page only looks at amino acids which contain a single -NH2 group and a single -COOH group. Amino acids as zwitterionsZwitterions in simple amino acid solutions An amino acid has both a basic amine group and an acidic carboxylic acid group.
There is an internal transfer of a hydrogen ion from the -COOH group to the -NH2 group to leave an ion with both a negative charge and a positive charge. This is called a zwitterion.
This is the form that amino acids exist in even in the solid state. If you dissolve the amino acid in water, a simple solution also contains this ion. A zwitterion is a compound with no overall electrical charge, but which contains separate parts which are positively and negatively charged. Adding an alkali to an amino acid solution If you increase the pH of a solution of an amino acid by adding hydroxide ions, the hydrogen ion is removed from the -NH3+ group.
You could show that the amino acid now existed as a negative ion using electrophoresis. In its simplest form, electrophoresis can just consist of a piece of moistened filter paper on a microscope slide with a crocodile clip at each end attached to a battery. A drop of amino acid solution is placed in the centre of the paper. Although the amino acid solution is colourless, its position after a time can be found by spraying it with a solution of ninhydrin. If the paper is allowed to dry and then heated gently, the amino acid shows up as a coloured spot. The amino acid would be found to travel towards the anode (the positive electrode). Adding an acid to an amino acid solution If you decrease the pH by adding an acid to a solution of an amino acid, the -COO- part of the zwitterion picks up a hydrogen ion.
This time, during electrophoresis, the amino acid would move towards the cathode (the negative electrode). Shifting the pH from one extreme to the other Suppose you start with the ion we've just produced under acidic conditions and slowly add alkali to it. That ion contains two acidic hydrogens - the one in the -COOH group and the one in the -NH3+ group. The more acidic of these is the one in the -COOH group, and so that is removed first - and you get back to the zwitterion.
So when you have added just the right amount of alkali, the amino acid no longer has a net positive or negative charge. That means that it wouldn't move towards either the cathode or anode during electrophoresis. The pH at which this lack of movement during electrophoresis happens is known as the isoelectric point of the amino acid. This pH varies from amino acid to amino acid. If you go on adding hydroxide ions, you will get the reaction we've already seen, in which a hydrogen ion is removed from the -NH3+ group.
|
|
|
Note: You might have expected the isoelectric point to be at pH 7 - when the solution is neither acidic nor alkaline. In fact, the isoelectric point for many amino acids is about pH 6. Explaining why it isn't at pH 7 is quite long-winded and almost certainly beyond what you will need for UK A level (or its equivalent) purposes. If you are interested, the problem is discussed at the bottom of this page. |
|
You can, of course, reverse the whole process by adding an acid to the ion we've just finished up with. That ion contains two basic groups - the -NH2 group and the -COO- group. The -NH2 group is the stronger base, and so picks up hydrogen ions first. That leads you back to the zwitterion again.
. . . and, of course, you can keep going by then adding a hydrogen ion to the -COO- group.
Why isn't the isoelectric point of an amino acid at pH 7? |
|
|
Warning: If you are a studying a UK-based syllabus, you are extremely unlikely to need this for exam purposes. It is included only for interest - you can probably safely ignore it. Check your syllabus and past papers. If you haven't got these, follow this link to find out how to get hold of them. |
|
When an amino acid dissolves in water, the situation is a little bit more complicated than we tend to pretend at this level. The zwitterion interacts with water molecules - acting as both an acid and a base. As an acid:
The -NH3+ group is a weak acid and donates a hydrogen ion to a water molecule. Because it is only a weak acid, the position of equilibrium will lie to the left. As a base:
The -COO- group is a weak base and takes a hydrogen ion from a water molecule. Again, the equilibrium lies to the left. When you dissolve an amino acid in water, both of these reactions are happening. But . . . The positions of the two equilibria aren't identical - they vary depending on the influence of the "R" group. In practice, for the simple amino acids we have been talking about, the position of the first equilibrium lies a bit further to the right than the second one. That means that there will be rather more of the negative ion from the amino acid in the solution than the positive one. In those circumstances, if you carried out electrophoresis on the unmodified solution, there would be a slight drift of amino acid towards the positive electrode (the anode). To stop that, you need to cut down the amount of the negative ion so that the concentrations of the two ions are identical. You can do that by adding a very small amount of acid to the solution, moving the position of the first equilibrium further to the left. Typically, the pH has to be lowered to about 6 to achieve this. For glycine, for example, the isoelectric point is pH 6.07; for alanine, 6.11; and for serine, 5.68. |
|
|
Note: I need to stress again that these figures (and the arguments) only hold where there is only one -NH2 group and one -COOH group in the amino acid. The isoelectric points are quite different if the molecule contains a second -NH2 or -COOH group. |
|
|
|