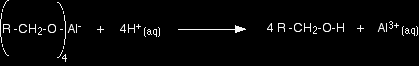REDUCTION OF CARBOXYLIC ACIDSThis page looks at the reduction of carboxylic acids to primary alcohols using lithium tetrahydridoaluminate(III) (lithium aluminium hydride), LiAlH4. The "(III)" is the oxidation state of the aluminium. Since aluminium only ever shows the +3 oxidation state in its compounds, the "(III)" is actually unnecessary. I shall leave it out for the rest of this page to make the name a bit shorter. |
|
|
Note: If you haven't come across this reducing agent before, you might find it useful to read a rather more detailed account of its use on the page about reduction of aldehydes and ketones. The present page isn't done in the same sort of detail as that one. Use the BACK button on your browser to return to this page. |
|
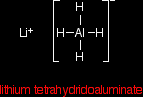
The reactionThe reducing agent Lithium tetrahydridoaluminate has the structure:
In the negative ion, one of the bonds is a co-ordinate covalent (dative covalent) bond using the lone pair on a hydride ion (H-) to form a bond with an empty orbital on the aluminium. |
|
|
Note: If you aren't happy about co-ordinate covalent (dative covalent) bonding you could follow this link, although it isn't important to understanding the rest of this page. Use the BACK button on your browser to return to this page. |
|
The reduction of a carboxylic acid The reaction happens in two stages - first to form an aldehyde and then a primary alcohol. Because lithium tetrahydridoaluminate reacts rapidly with aldehydes, it is impossible to stop at the halfway stage. Equations for these reactions are usually written in a simplified form for UK A level purposes. The "[H]" in the equations represents hydrogen from a reducing agent. Because of the impossibility of stopping at the aldehyde, there isn't much point in giving an equation for the two separate stages. The overall reaction is:
"R" is hydrogen or a hydrocarbon group. For example, ethanoic acid will reduce to the primary alcohol, ethanol.
|
|
|
Note: Follow this link if you aren't sure what a primary alcohol is. Use the BACK button on your browser to return to this page. |
|
Sodium tetrahydridoborate (sodium borohydride) won't work! If you are familiar with the reduction of aldehydes and ketones using lithium tetrahydridoaluminate, you are probably aware that sodium tetrahydridoborate is often used as a safer alternative. It CAN'T be used with carboxylic acids. The sodium tetrahydridoborate isn't reactive enough to reduce carboxylic acids. Reaction conditions Lithium tetrahydridoaluminate reacts violently with water and so the reactions are carried out in solution in dry ethoxyethane (diethyl ether or just "ether"). The reaction happens at room temperature. At the end of the reaction, the product is a complex aluminium salt. This is converted into the alcohol by treatment with dilute sulphuric acid.
|
|
|
Note: You are unlikely to need this equation for UK A level purposes, although you may need to know that you have to finish the reaction off using dilute sulphuric acid. |
|
|
|