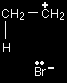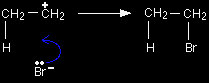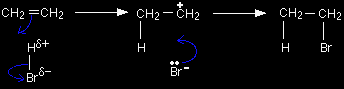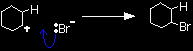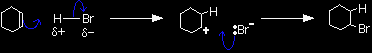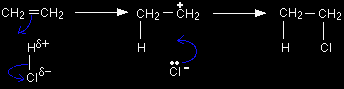EXPLAINING THE REACTION BETWEEN SYMMETRICAL ALKENES AND THE HYDROGEN HALIDESThis page guides you through the mechanism for the electrophilic addition of hydrogen halides such as hydrogen bromide with symmetrical alkenes like ethene or cyclohexene. Unsymmetrical alkenes are covered separately, and you will find a link at the bottom of the page. Electrophilic addition reactions involving hydrogen bromide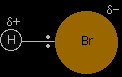
Hydrogen bromide is chosen as a typical hydrogen halide. Bromine is more electronegative than hydrogen. That means that the bonding pair of electrons is pulled towards the bromine end of the bond, and so the hydrogen bromide molecule is polar. |
|||||||||||||||||
|
Note: If you aren't sure about electronegativity and bond polarity follow this link before you read on. Use the BACK button on your browser to return to this page. |
|||||||||||||||||
The reaction of ethene with hydrogen bromide 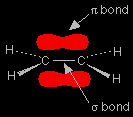
The structure of ethene is shown in the diagram on the right. The pi bond is an orbital above and below the plane of the rest of the molecule, and relatively exposed to things around it. The two electrons in this orbital are highly attractive to anything which is positively charged. |
|||||||||||||||||
|
Note: If you aren't sure about this, it would be a good idea to read the introductory page on electrophilic addition before you go on. Use the BACK button on your browser to return to this page. |
|||||||||||||||||
The slightly positive hydrogen atom in the hydrogen bromide acts as an electrophile, and is strongly attracted to the electrons in the pi bond. |
|||||||||||||||||
|
Electrophile: A substance with a strong attraction to a negative region in another substance. Electrophiles are either fully positive ions, or the slightly positive end of a polar molecule. |
|||||||||||||||||
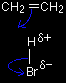
The electrons from the pi bond move down towards the slightly positive hydrogen atom. In the process, the electrons in the H-Br bond are repelled down until they are entirely on the bromine atom, producing a bromide ion. |
|||||||||||||||||
|
Help! If you aren't sure about the use of curly arrows in mechanisms, you must follow this link before you go on. Use the BACK button on your browser to return to this page. |
|||||||||||||||||
That leaves you with these two ions at this half-way stage of the reaction:
The ion with a positive charge on the carbon atom is called a carbocation or carbonium ion (an older term). Why is there a positive charge on the carbon atom? The pi bond was originally made up of an electron from each of the carbon atoms. Both of those electrons have been used to make a new bond to the hydrogen. That leaves the right-hand carbon an electron short - hence positively charged. In the second stage of the mechanism, the lone pair of electrons on the bromide ion is strongly attracted to the positive carbon and moves towards it until a bond is formed.
|
|||||||||||||||||
|
Note: For clarity only one of the lone pairs around the bromide ion is shown. That's perfectly acceptable, because the other three lone pairs aren't involved in the process - they are pointing in the wrong directions. |
|||||||||||||||||
The overall mechanism is therefore
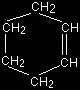
The reaction of cyclohexene with hydrogen bromide Cyclohexene is chosen an an example of a fairly commonly used symmetrical alkene. The fact that it is a ring structure doesn't make any difference to the mechanism. The full structure of cyclohexene is
but it is often abbreviated to
In this diagram, there is a carbon atom at each corner, and enough hydrogens attached to each carbon to bring the total number of bonds per carbon atom up to 4. The double bond has an easily attacked pi bond exactly as in ethene, and the electrons in that bond are attracted towards the slightly positive hydrogen atom in the HBr. Once again, the pi bond electrons swing to make a bond with the hydrogen, and push the electrons in the H-Br bond fully onto the bromine, making a bromide ion.
|
|||||||||||||||||
|
Care! Think carefully about which way the pi bond electrons swing. In this case, think of them as being pivotted about the top carbon atom. It is therefore that carbon atom which is joined to the new hydrogen. |
|||||||||||||||||
In the second stage, one of the lone pairs of electrons on the bromide ion is attracted to the positively charged carbon atom and forms a bond with it.
The overall mechanism is therefore:
Electrophilic addition reactions involving the other hydrogen halidesThe mechanisms The other hydrogen halides behave in exactly the same way as hydrogen bromide. For example, compare the reaction between ethene and hydrogen bromide with the one between ethene and hydrogen chloride.
There's no need to learn both mechanisms. As long as you know one of them, all you have to do is swap one halogen atom for another. That's equally true for hydrogen fluoride or hydrogen iodide. The different rates of reaction The rate of reaction increases as you go from HF to HCl to HBr to HI.
The reason for this is that as the halogen atoms get bigger, the strength of the hydrogen-halogen bond falls. Bond strengths (measured in kilojoules per mole) are:
In the first step of these mechanisms, the hydrogen-halogen bond breaks as the electron pair is forced down onto the halogen atom. Breaking bonds needs energy, and if the bond is weaker, it will break more easily - needing less energy. That means that the activation energy for the reactions will fall as you go from hydrogen fluoride to hydrogen iodide. The lower the activation energy, the faster the reaction. |
|||||||||||||||||
|
Activation energy: The minimum energy needed before a reaction will occur. In this case it is the energy needed to break the various bonds and make the carbocation and the halide ion. |
|||||||||||||||||
Beware! A red herring!
People sometimes get confused because there is another tempting place to look for the reason why the reaction rates are different between the various hydrogen halides. The halogens have different electronegativities - with fluorine being the most electronegative and iodine the least. That means that the hydrogen in HF will have the greatest positive charge and so will be attracted most strongly to the pi bond.
It would be tempting to think that that would produce the fastest reaction - but not so! Although the HF may well be attracted most strongly, attraction alone isn't enough. If anything is to happen, bonds have to be broken - and here HF is at a disadvantage, because the bond is very strong. The lesson from all this When you are trying to find reasons for differing rates of reactions, always look first at differences in bond strengths. Electronegativity differences may be interesting, but rarely give you the answer you want! |
|||||||||||||||||