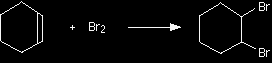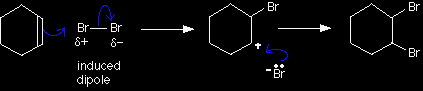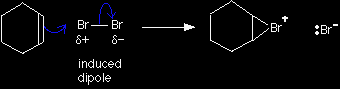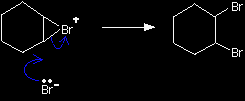THE REACTION BETWEEN SYMMETRICAL ALKENES AND BROMINE
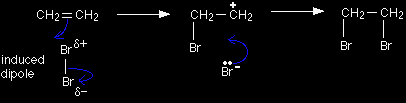
This page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between bromine (and the other halogens) and alkenes like ethene and cyclohexene. If you want the mechanisms explained to you in detail, there is a link at the bottom of the page. The electrophilic addition of bromine to etheneThe facts Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The double bond breaks, and a bromine atom becomes attached to each carbon. The bromine loses its original red-brown colour to give a colourless liquid. In the case of the reaction with ethene, 1,2-dibromoethane is formed.
This decolourisation of bromine is often used as a test for a carbon-carbon double bond. If an aqueous solution of bromine is used ("bromine water"), you get a mixture of products. The presence of the water complicates the mechanism beyond what is required by current UK A level (or equivalent) syllabuses. The other halogens, apart from fluorine, behave similarly. (Fluorine reacts explosively with all hydrocarbons - including alkenes - to give carbon and hydrogen fluoride.) If you are interested in the reaction with, say, chlorine, all you have to do is to replace Br by Cl in all the equations on this page. The mechanism for the reaction between ethene and bromine The reaction is an example of electrophilic addition. |
|
|
Warning! There are two versions of the ethene / bromine mechanism in common use, and you must know which your examiners will accept. One version is simplified to bring it into line with the other alkene electrophilic addition mechanisms. You will probably find that your examiners will accept this one, but you must find out to be sure. You almost certainly won't be able to tell this from your syllabus. You need to refer to recent mark schemes, or to any support material that your examiners provide. If you still aren't sure, contact your examiners direct. If you are working towards a UK-based exam, you can find out how to do this by using the link to your Board's web site on the syllabuses page. The person you need to contact will probably have the title Subject Officer for Chemistry or something similar. Ask whether they want the mechanism for the reaction between bromine and alkenes which proceeds via a carbocation or via a bromonium ion intermediate. |
|
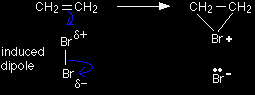
Bromine as an electrophile The bromine is a very "polarisable" molecule and the approaching pi bond in the ethene induces a dipole in the bromine molecule. If you draw this mechanism in an exam, write the words "induced dipole" next to the bromine molecule - to show that you understand what's going on. The simplified version of the mechanism |
|
|
Note: Use this version unless your examiners insist on the more accurate one. |
|
The more accurate version of the mechanism |
|
|
Note: Don't learn this unless you have to. There is a real risk of getting confused. If your examiners are happy to accept the simple version, there's no point in making life difficult for yourself. |
|
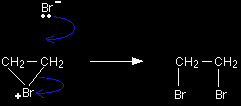
In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. A bromonium ion is formed.
The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction.
The electrophilic addition of bromine to cyclohexeneThe facts Cyclohexene reacts with bromine in the same way and under the same conditions as any other alkene. 1,2-dibromocyclohexane is formed.
The mechanism for the reaction between cyclohexene and bromine The reaction is an example of electrophilic addition. |
|
|
Warning! Again, there are two versions of this mechanism in common use, and you must know which your examiners will accept. |
|
Bromine as an electrophile Again, the bromine is polarised by the approaching pi bond in the cyclohexene. Don't forget to write the words "induced dipole" next to the bromine molecule. The simplified version of the mechanism |
|
|
Note: Use this version unless your examiners insist on the more accurate one. |
|
The alternative version of the mechanism |
|
|
Note: Don't learn this unless you have to. If your examiners are happy to accept the simple version, there's no point in making life difficult for yourself. |
|
In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. A bromonium ion is formed.
The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction.
|
|