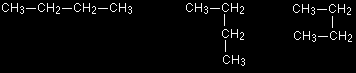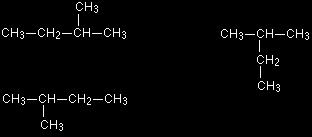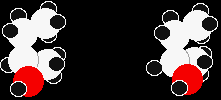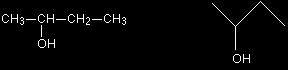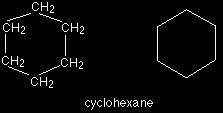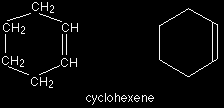DRAWING ORGANIC MOLECULESThis page explains the various ways that organic molecules can be represented on paper or on screen - including molecular formulae, and various forms of structural formulae. Molecular formulaeA molecular formula simply counts the numbers of each sort of atom present in the molecule, but tells you nothing about the way they are joined together. For example, the molecular formula of butane is C4H10, and the molecular formula of ethanol is C2H6O. Molecular formulae are very rarely used in organic chemistry, because they don't give any useful information about the bonding in the molecule. About the only place where you might come across them is in equations for the combustion of simple hydrocarbons, for example:
In cases like this, the bonding in the organic molecule isn't important. Structural formulaeA structural formula shows how the various atoms are bonded. There are various ways of drawing this and you will need to be familiar with all of them. Displayed formulae A displayed formula shows all the bonds in the molecule as individual lines. You need to remember that each line represents a pair of shared electrons. For example, this is a model of methane together with its displayed formula:
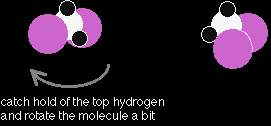
Notice that the way the methane is drawn bears no resemblance to the actual shape of the molecule. Methane isn't flat with 90° bond angles. This mismatch between what you draw and what the molecule actually looks like can lead to problems if you aren't careful. For example, consider the simple molecule with the molecular formula CH2Cl2. You might think that there were two different ways of arranging these atoms if you drew a displayed formula.
The chlorines could be opposite each other or at right angles to each other. But these two structures are actually exactly the same. Look at how they appear as models.
One structure is in reality a simple rotation of the other one. |
|
|
Note: This is all much easier to understand if you have actually got some models to play with. If your school or college hasn't given you the opportunity to play around with molecular models in the early stages of your organic chemistry course, you might consider getting hold of a cheap set. The models made by Molymod are both cheap and easy to use. An introductory organic set is more than adequate. Google molymod to find a supplier and more about them, or click on the picture or text link below to see a typical example from Amazon. (Don't click on the "Buy" button unless you really want to buy it!) Share the cost with some friends, keep it in good condition and don't lose any bits, and resell it via eBay or Amazon at the end of your course. Alternatively, get hold of some coloured Plasticene (or other children's modelling clay) and some used matches and make your own. It's cheaper, but more difficult to get the bond angles right. |
|
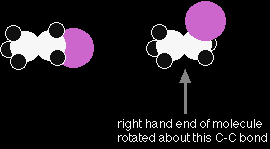
Consider a slightly more complicated molecule, C2H5Cl. The displayed formula could be written as either of these:
But, again these are exactly the same. Look at the models.
The commonest way to draw structural formulae For anything other than the most simple molecules, drawing a fully displayed formula is a bit of a bother - especially all the carbon-hydrogen bonds. You can simplify the formula by writing, for example, CH3 or CH2 instead of showing all these bonds.
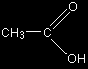
So for example, ethanoic acid would be shown in a fully displayed form and a simplified form as:
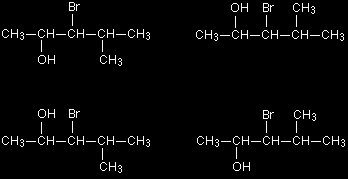
You could even condense it further to CH3COOH, and would probably do this if you had to write a simple chemical equation involving ethanoic acid. You do, however, lose something by condensing the acid group in this way, because you can't immediately see how the bonding works. You still have to be careful in drawing structures in this way. Remember from above that these two structures both represent the same molecule:
The next three structures all represent butane.
All of these are just versions of four carbon atoms joined up in a line. The only difference is that there has been some rotation about some of the carbon-carbon bonds. You can see this in a couple of models.
Not one of the structural formulae accurately represents the shape of butane. The convention is that we draw it with all the carbon atoms in a straight line - as in the first of the structures above. This is even more important when you start to have branched chains of carbon atoms. The following structures again all represent the same molecule - 2-methylbutane.
The two structures on the left are fairly obviously the same - all we've done is flip the molecule over. The other one isn't so obvious until you look at the structure in detail. There are four carbons joined up in a row, with a CH3 group attached to the next-to-end one. That's exactly the same as the other two structures. If you had a model, the only difference between these three diagrams is that you have rotated some of the bonds and turned the model around a bit. To overcome this possible confusion, the convention is that you always look for the longest possible chain of carbon atoms, and then draw it horizontally. Anything else is simply hung off that chain. It doesn't matter in the least whether you draw any side groups pointing up or down. All of the following represent exactly the same molecule.
If you made a model of one of them, you could turn it into any other one simply by rotating one or more of the carbon-carbon bonds. How to draw structural formulae in 3-dimensions There are occasions when it is important to be able to show the precise 3-D arrangement in parts of some molecules. To do this, the bonds are shown using conventional symbols:
For example, you might want to show the 3-D arrangement of the groups around the carbon which has the -OH group in butan-2-ol. Butan-2-ol has the structural formula:
Using conventional bond notation, you could draw it as, for example:
The only difference between these is a slight rotation of the bond between the centre two carbon atoms. This is shown in the two models below. Look carefully at them - particularly at what has happened to the lone hydrogen atom. In the left-hand model, it is tucked behind the carbon atom. In the right-hand model, it is in the same plane. The change is very slight.
It doesn't matter in the least which of the two arrangements you draw. You could easily invent other ones as well. Choose one of them and get into the habit of drawing 3-dimensional structures that way. My own habit (used elsewhere on this site) is to draw two bonds going back into the paper and one coming out - as in the left-hand diagram above. Notice that no attempt was made to show the whole molecule in 3-dimensions in the structural formula diagrams. The CH2CH3 group was left in a simple form. Keep diagrams simple - trying to show too much detail makes the whole thing amazingly difficult to understand! Skeletal formulae In a skeletal formula, all the hydrogen atoms are removed from carbon chains, leaving just a carbon skeleton with functional groups attached to it. For example, we've just been talking about butan-2-ol. The normal structural formula and the skeletal formula look like this:
In a skeletal diagram of this sort
Beware! Diagrams of this sort take practice to interpret correctly - and may well not be acceptable to your examiners (see below). There are, however, some very common cases where they are frequently used. These cases involve rings of carbon atoms which are surprisingly awkward to draw tidily in a normal structural formula. Cyclohexane, C6H12, is a ring of carbon atoms each with two hydrogens attached. This is what it looks like in both a structural formula and a skeletal formula.
And this is cyclohexene, which is similar but contains a double bond:
But the commonest of all is the benzene ring, C6H6, which has a special symbol of its own.
|
|
|
Note: Explaining exactly what this structure means needs more space than is available here. It is explained in full in two pages on the structure of benzene elsewhere in this site. It would probably be better not to follow this link unless you are actively interested in benzene chemistry at the moment - it will lead you off into quite deep water! |
|
Deciding which sort of formula to useThere's no easy, all-embracing answer to this problem. It depends more than anything else on experience - a feeling that a particular way of writing a formula is best for the situation you are dealing with. Don't worry about this - as you do more and more organic chemistry, you will probably find it will come naturally. You'll get so used to writing formulae in reaction mechanisms, or for the structures for isomers, or in simple chemical equations, that you won't even think about it.
There are, however, a few guidelines that you should follow. What does your syllabus say? Different examiners will have different preferences. Check first with your syllabus. If you've down-loaded a copy of your syllabus from your examiners' web site, it is easy to check what they say they want. Use the "find" function on your Adobe Acrobat Reader to search the organic section(s) of the syllabus for the word "formula". You should also check recent exam papers and (particulary) mark schemes to find out what sort of formula the examiners really prefer in given situations. You could also look at any support material published by your examiners. |
|
|
Note: If you are working to a UK-based syllabus and haven't got a copy of that syllabus and recent exam papers, follow this link to find out how to get them. |
|
What if you still aren't sure? Draw the most detailed formula that you can fit into the space available. If in doubt, draw a fully displayed formula. You would never lose marks for giving too much detail. Apart from the most trivial cases (for example, burning hydrocarbons), never use a molecular formula. Always show the detail around the important part(s) of a molecule. For example, the important part of an ethene molecule is the carbon-carbon double bond - so write (at the very least) CH2=CH2 and not C2H4. Where a particular way of drawing a structure is important, this will always be pointed out where it arises elsewhere on this site.
|
|