THE FINGERPRINT REGION OF AN INFRA-RED SPECTRUMThis page explains what the fingerprint region of an infra-red spectrum is, and how it can be used to identify an organic molecule. |
|
|
Note: It would be helpful if you first read the introductory page on infra-red spectra if you haven't already done so. |
|
What is the fingerprint region This is a typical infra-red spectrum:
|
|
|
Note: The infra-red spectra on this page have been produced from graphs taken from the Spectral Data Base System for Organic Compounds (SDBS) at the National Institute of Materials and Chemical Research in Japan. It is possible that small errors may have been introduced during the process of converting them for use on this site, but these won't affect the argument in any way. |
|
Each trough is caused because energy is being absorbed from that particular frequency of infra-red radiation to excite bonds in the molecule to a higher state of vibration - either stretching or bending. Some of the troughs are easily used to identify particular bonds in a molecule. For example, the big trough at the left-hand side of the spectrum is used to identify the presence of an oxygen-hydrogen bond in an -OH group. |
|
|
Note: Using troughs in this way to identify particular bonds is covered on a separate page. |
|
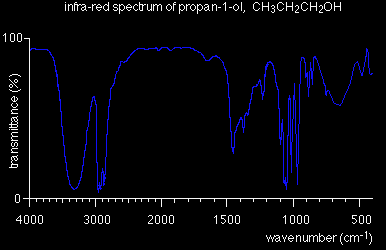
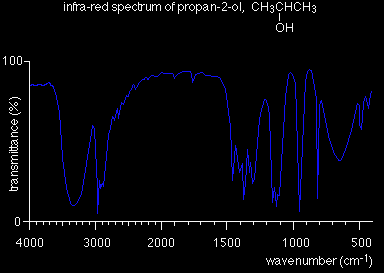
The region to the right-hand side of the diagram (from about 1500 to 500 cm-1) usually contains a very complicated series of absorptions. These are mainly due to all manner of bending vibrations within the molecule. This is called the fingerprint region. It is much more difficult to pick out individual bonds in this region than it is in the "cleaner" region at higher wavenumbers. The importance of the fingerprint region is that each different compound produces a different pattern of troughs in this part of the spectrum. Using the fingerprint region Compare the infra-red spectra of propan-1-ol and propan-2-ol. Both compounds contain exactly the same bonds. Both compounds have very similar troughs in the area around 3000 cm-1 - but compare them in the fingerprint region between 1500 and 500 cm-1.
The pattern in the fingerprint region is completely different and could therefore be used to identify the compound. So . . . to positively identify an unknown compound, use its infra-red spectrum to identify what sort of compound it is by looking for specific bond absorptions. That might tell you, for example, that you had an alcohol because it contained an -OH group. You would then compare the fingerprint region of its infra-red spectrum with known spectra measured under exactly the same conditions to find out which alcohol (or whatever) you had.
|
|